Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- PMID: 29494592
- PMCID: PMC5832187
- DOI: 10.1371/journal.pmed.1002520
Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
Abstract
Background: Few medicines have been approved for children, leading to rates of off-label prescribing reported to be as high as 90%. In 2007, the European Union adopted the Paediatric Regulation, which mandates that pharmaceutical companies conduct paediatric studies for all new medicines, unless granted a waiver. We aimed to evaluate the availability of paediatric trial results from studies required under the Paediatric Regulation for new medicines authorised in the EU.
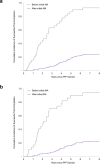
Methods and findings: The European Medicines Agency (EMA) public database of paediatric investigation plans was searched for new medicines centrally authorised in the EU between 1 January 2010 and 31 December 2014 with at least 1 required paediatric study. For our study cohort of paediatric clinical trials required for these medicines, we used internal EMA databases and publicly available trial registries to determine changes to the planned completion date or study design, rates of trial completion, time to trial completion, and results reporting (peer-reviewed publication or posting on trial registry). Cox proportional hazards regression models were constructed to examine factors associated with study completion. A total of 326 paediatric clinical trials were required for 122 novel medicines authorised by the EMA between 2010 and 2014. In all, 76% (247/326) of paediatric studies were not planned to be completed until after the initial marketing authorisation. The planned completion dates for 50% (162/326) were further postponed by a median of 2.2 years. Overall, 38% (124/326) of paediatric studies were completed as of 30 November 2017. The rate of trial completion for paediatric studies planned to be completed after initial marketing authorisation was 23% (56/247), versus 86% (68/79) for trials planned to be completed before authorisation (adjusted hazard ratio 0.11; 95% CI 0.06-0.19). Among completed studies, the results were reported in a public registry or in the peer-reviewed literature for 85% (105/124) at a median of 1.1 years after study completion, and 60% (74/124) were published in a peer-reviewed journal. Limitations of this study include the potential lack of generalisability to medicines not authorised by the EMA and the possibility for more of these trials to be completed or published in the future.
Conclusions: The completion of many paediatric studies required under the Paediatric Regulation has been delayed. Paediatric studies planned to be completed after marketing authorisation were associated with a lower likelihood of eventual completion, highlighting the need to examine the implementation of current policies in ensuring timely availability of important paediatric information.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: TJH reports prior employment by Blackstone and Bain Capital, which have invested in healthcare companies.
Figures

References
-
- Weda M, Hoebert J, Vervloet M, Moltó Puigmarti C, Damen N, Marchange S, et al. Study on off-label use of medicinal products in the European Union. Brussels: European Union; 2017. [cited 2018 Feb 8]. Available from: https://ec.europa.eu/health/sites/health/files/files/documents/2017_02_2....
-
- McLay JS, Tanaka M, Ekins-Daukes S, Helms PJ. A prospective questionnaire assessment of attitudes and experiences of off label prescribing among hospital based paediatricians. Arch Dis Child. 2006;91(7):584–7. doi: 10.1136/adc.2005.081828 - DOI - PMC - PubMed
-
- European Medicines Agency. Paroxetine. London: European Medicines Agency; 2018. [cited 2018 Feb 12]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referr....
-
- Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. Official Journal of the European Union. L 378/1. 2006 Dec 27.
-
- Regulation (EC) No 1902/2006 of the European Parliament and of the Council of 20 December 2006 amending Regulation 1901/2006 on medicinal products for paediatric use. Official Journal of the European Union. L 378/20. 2006 Dec 27.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

