The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- PMID: 29494593
- PMCID: PMC5832192
- DOI: 10.1371/journal.pmed.1002514
The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
Abstract
Background: Globally, the population of adolescents living with perinatally acquired HIV (APHs) continues to expand. In this study, we pooled data from observational pediatric HIV cohorts and cohort networks, allowing comparisons of adolescents with perinatally acquired HIV in "real-life" settings across multiple regions. We describe the geographic and temporal characteristics and mortality outcomes of APHs across multiple regions, including South America and the Caribbean, North America, Europe, sub-Saharan Africa, and South and Southeast Asia.
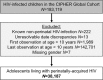
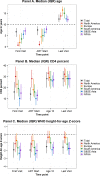
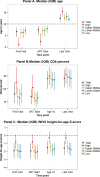
Methods and findings: Through the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER), individual retrospective longitudinal data from 12 cohort networks were pooled. All children infected with HIV who entered care before age 10 years, were not known to have horizontally acquired HIV, and were followed up beyond age 10 years were included in this analysis conducted from May 2016 to January 2017. Our primary analysis describes patient and treatment characteristics of APHs at key time points, including first HIV-associated clinic visit, antiretroviral therapy (ART) start, age 10 years, and last visit, and compares these characteristics by geographic region, country income group (CIG), and birth period. Our secondary analysis describes mortality, transfer out, and lost to follow-up (LTFU) as outcomes at age 15 years, using competing risk analysis. Among the 38,187 APHs included, 51% were female, 79% were from sub-Saharan Africa and 65% lived in low-income countries. APHs from 51 countries were included (Europe: 14 countries and 3,054 APHs; North America: 1 country and 1,032 APHs; South America and the Caribbean: 4 countries and 903 APHs; South and Southeast Asia: 7 countries and 2,902 APHs; sub-Saharan Africa, 25 countries and 30,296 APHs). Observation started as early as 1982 in Europe and 1996 in sub-Saharan Africa, and continued until at least 2014 in all regions. The median (interquartile range [IQR]) duration of adolescent follow-up was 3.1 (1.5-5.2) years for the total cohort and 6.4 (3.6-8.0) years in Europe, 3.7 (2.0-5.4) years in North America, 2.5 (1.2-4.4) years in South and Southeast Asia, 5.0 (2.7-7.5) years in South America and the Caribbean, and 2.1 (0.9-3.8) years in sub-Saharan Africa. Median (IQR) age at first visit differed substantially by region, ranging from 0.7 (0.3-2.1) years in North America to 7.1 (5.3-8.6) years in sub-Saharan Africa. The median age at ART start varied from 0.9 (0.4-2.6) years in North America to 7.9 (6.0-9.3) years in sub-Saharan Africa. The cumulative incidence estimates (95% confidence interval [CI]) at age 15 years for mortality, transfers out, and LTFU for all APHs were 2.6% (2.4%-2.8%), 15.6% (15.1%-16.0%), and 11.3% (10.9%-11.8%), respectively. Mortality was lowest in Europe (0.8% [0.5%-1.1%]) and highest in South America and the Caribbean (4.4% [3.1%-6.1%]). However, LTFU was lowest in South America and the Caribbean (4.8% [3.4%-6.7%]) and highest in sub-Saharan Africa (13.2% [12.6%-13.7%]). Study limitations include the high LTFU rate in sub-Saharan Africa, which could have affected the comparison of mortality across regions; inclusion of data only for APHs receiving ART from some countries; and unavailability of data from high-burden countries such as Nigeria.
Conclusion: To our knowledge, our study represents the largest multiregional epidemiological analysis of APHs. Despite probable under-ascertained mortality, mortality in APHs remains substantially higher in sub-Saharan Africa, South and Southeast Asia, and South America and the Caribbean than in Europe. Collaborations such as CIPHER enable us to monitor current global temporal trends in outcomes over time to inform appropriate policy responses.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: AS's institution receives research and travel funding from ViiV Healthcare. CT has participated in an advisory board for ViiV Healthcare and received research grants from the European Commission, UK Medical Research Council, Public Health England, PENTA Foundation, Abbvie, and ViiV. CT is on the Advisory Board of the Antiretroviral Pregnancy Registry, the Board of the PENTA Foundation, and the UK Infectious Diseases in Pregnancy Screening Advisory Group. MV's work at CIPHER is funded through Unrestricted Educational grants received from ViiV Healthcare and Janssen to the International AIDS Society. CS has received personal payment for preparation of educational materials for Gilead Sciences and ViiV Healthcare. JW's institution has received academic grants from the INSERM-ANRS for cohorts of JW's responsibility involved in the study. SW receives a fee from Baylor International Pediatric AIDS Initiative for consultancy services related to research. MJA receives funding from NIH to perform research in the IMPAACT network. LGB is guest editor for the
Figures
References
-
- UNAIDS. UNAIDS 2016 Estimates, 2017 [cited 2017 December 27]. Available from: http://aidsinfo.unaids.org/.
-
- World Health Organization, Health for the world's adolescents: a second chance in the second decade Geneva, Switzerland: World Health Organization; 2014. [cited 2017 January 06]. Available from: http://apps.who.int/adolescent/second-decade/files/1612_MNCAH_HWA_Execut....
-
- Davies MA, Gibb D, Turkova A. Survival of HIV-1 vertically infected children. Curr Opin HIV AIDS. 2016;11(5):455–64. doi: 10.1097/COH.0000000000000303 - DOI - PMC - PubMed
-
- Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45(7):918–24. doi: 10.1086/521167 - DOI - PubMed
-
- Johnson LF, Davies MA, Moultrie H, Sherman GG, Bland RM, Rehle TM, et al. The effect of early initiation of antiretroviral treatment in infants on pediatric AIDS mortality in South Africa: a model-based analysis. Pediatr Infect Dis J. 2012;31(5):474–80. doi: 10.1097/INF.0b013e3182456ba2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HD052104/HD/NICHD NIH HHS/United States
- MC_UU_12023/17/MRC_/Medical Research Council/United Kingdom
- U01 AI096299/AI/NIAID NIH HHS/United States
- U01 AI069923/AI/NIAID NIH HHS/United States
- PEPFAR/PEPFAR/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- U01 AI069919/AI/NIAID NIH HHS/United States
- U2G PS001537/PS/NCHHSTP CDC HHS/United States
- 001/WHO_/World Health Organization/International
- U01 AI069924/AI/NIAID NIH HHS/United States
- MC_UU_12023/26/MRC_/Medical Research Council/United Kingdom
- UM1 AI068632/AI/NIAID NIH HHS/United States
- U01 AI069911/AI/NIAID NIH HHS/United States
- U62 PS223540/PS/NCHHSTP CDC HHS/United States
- U01 AI069907/AI/NIAID NIH HHS/United States
- U01 HD052102/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

