Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation
- PMID: 29494886
- PMCID: PMC7125521
- DOI: 10.1016/j.bios.2018.02.040
Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation
Abstract
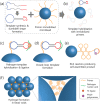
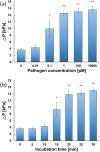
There has been an urgent need to quickly screen and isolate patients with viral infections from patients with similar symptoms at point-of-care. In this study, we introduce a new microfluidic method for detection of various viruses using rolling circle amplification (RCA) of pathogens on the surface of thousands of microbeads packed in microchannels. When a targeted pathogen meets the corresponding particular template, the DNAs are rapidly amplified into a specific dumbbell shape through the RCA process, forming a DNA hydrogel and blocking the flow path formed between the beads. Due to the significant increase in reaction surface area, the detection time was shortened to less than 15 min and the detection limit of various pathogens has been reached to 0.1 pM. By injecting the stained liquid, the existence of the target pathogens in a sample fluid can be determined with the naked eye. Furthermore, by integrating multi-channel design, simultaneous phenotyping of various infective pathogens (i.e., Ebola, Middle East respiratory syndrome (MERS), and others) in biological specimens can be performed at a point-of-care.
Keywords: DNA; Hydrogel; Microbead; Microfluidics; Molecular diagnosis; RCA.
Copyright © 2018 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Allegranzi B., Bagheri Nejad S., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D. Lancet. 2011;377:228–241. - PubMed
-
- Demidov V.V. Expert Rev. Mol. Diagn. 2002;2:542–548. - PubMed
-
- Gan S.D., Patel K.R. J. Investig. Dermatol. 2013;133:e12. - PubMed
-
- Jarvius J., Melin J., Goransson J., Stenberg J., Fredriksson S., Gonzalez-Rey C., Bertilsson S., Nilsson M. Nat. Methods. 2006;3:725–727. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

