Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold
- PMID: 29495264
- PMCID: PMC5853755
- DOI: 10.3390/nano8020124
Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold
Abstract
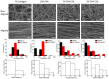
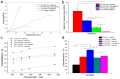
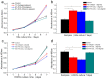
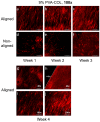
Corneal diseases are the main reason of vision loss globally. Constructing a corneal equivalent which has a similar strength and transparency with the native cornea, seems to be a feasible way to solve the shortage of donated cornea. Electrospun collagen scaffolds are often fabricated and used as a tissue-engineered cornea, but the main drawback of poor mechanical properties make it unable to meet the requirement for surgery suture, which limits its clinical applications to a large extent. Aligned polyvinyl acetate (PVA)/collagen (PVA-COL) scaffolds were electrospun by mixing collagen and PVA to reinforce the mechanical strength of the collagen electrospun scaffold. Human keratocytes (HKs) and human corneal epithelial cells (HCECs) inoculated on aligned and random PVA-COL electrospun scaffolds adhered and proliferated well, and the aligned nanofibers induced orderly HK growth, indicating that the designed PVA-COL composite nanofibrous electrospun scaffold is suitable for application in tissue-engineered cornea.
Keywords: corneal tissue; electrospun; nanofibrous scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wilson S.L., Wimpenny I., Ahearne M., Rauz S., Haj A.J.E., Yang Y. Chemical and Topographical Effects on Cell Differentiation and Matrix Elasticity in a Corneal Stromal Layer Model. Adv. Funct. Mater. 2012;22:3641–3649. doi: 10.1002/adfm.201200655. - DOI
-
- World Health Organization . Draft Action Plan for the Prevention of Avoidable Blindness and Visual Impairment 2014–2019: Towards Universal Eye Health: A Global Action Plan 2014–2019. Sixty-Sixth World Health Assembly; Geneva, Switzerland: 2013. Report by the Secretariat.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

