In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway
- PMID: 29495362
- PMCID: PMC5872681
- DOI: 10.3390/nu10030263
In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway
Abstract
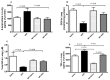
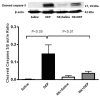
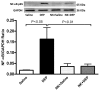
Numerous studies have shown that acute particulate air pollution exposure is linked with pulmonary adverse effects, including alterations of pulmonary function, inflammation, and oxidative stress. Nootkatone, a constituent of grapefruit, has antioxidant and anti-inflammatory effects. However, the effect of nootkatone on lung toxicity has not been reported so far. In this study we evaluated the possible protective effects of nootkatone on diesel exhaust particles (DEP)-induced lung toxicity, and the possible mechanisms underlying these effects. Mice were intratracheally (i.t.) instilled with either DEP (30 µg/mouse) or saline (control). Nootkatone was given to mice by gavage, 1 h before i.t. instillation, with either DEP or saline. Twenty-four hours following DEP exposure, several physiological and biochemical endpoints were assessed. Nootkatone pretreatment significantly prevented the DEP-induced increase in airway resistance in vivo, decreased neutrophil infiltration in bronchoalveolar lavage fluid, and abated macrophage and neutrophil infiltration in the lung interstitium, assessed by histolopathology. Moreover, DEP caused a significant increase in lung concentrations of 8-isoprostane and tumor necrosis factor α, and decreased the reduced glutathione concentration and total nitric oxide activity. These actions were all significantly alleviated by nootkatone pretreatment. Similarly, nootkatone prevented DEP-induced DNA damage and prevented the proteolytic cleavage of caspase-3. Moreover, nootkatone inhibited nuclear factor-kappaB (NF-κB) induced by DEP. We conclude that nootkatone prevented the DEP-induced increase in airway resistance, lung inflammation, oxidative stress, and the subsequent DNA damage and apoptosis through a mechanism involving inhibition of NF-κB activation. Nootkatone could possibly be considered a beneficial protective agent against air pollution-induced respiratory adverse effects.
Keywords: Lung; NF-κB; airway resistance; diesel exhaust particles; inflammation; nootkatone; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cai Y., Zhang B., Ke W., Feng B., Lin H., Xiao J., Zeng W., Li X., Tao J., Yang Z., et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants with Hypertension: A Systematic Review and Meta-Analysis. Hypertension. 2016;68:62–70. doi: 10.1161/HYPERTENSIONAHA.116.07218. - DOI - PubMed
-
- Salvi S., Blomberg A., Rudell B., Kelly F., Sandstrom T., Holgate S.T., Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

