Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions
- PMID: 29495363
- PMCID: PMC5872913
- DOI: 10.3390/ma11030334
Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions
Abstract
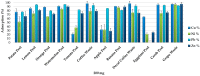
Recent studies have shown the potential of food waste materials as low cost adsorbents for the removal of heavy metals and toxic elements from wastewater. However, the adsorption experiments have been performed in heterogeneous conditions, consequently it is difficult to compare the efficiency of the individual adsorbents. In this study, the adsorption capacities of 12 food waste materials were evaluated by comparing the adsorbents' efficiency for the removal of 23 elements from complex multi-element solutions, maintaining homogeneous experimental conditions. The examined materials resulted to be extremely efficient for the adsorption of many elements from synthetic multi-element solutions as well as from a heavy metal wastewater. The 12 adsorbent surfaces were analyzed by Fourier transform infrared spectroscopy and showed different types and amounts of functional groups, which demonstrated to act as adsorption active sites for various elements. By multivariate statistical computations of the obtained data, the 12 food waste materials were grouped in five clusters characterized by different elements' removal efficiency which resulted to be in correlation with the specific adsorbents' chemical structures. Banana peel, watermelon peel and grape waste resulted the least selective and the most efficient food waste materials for the removal of most of the elements.
Keywords: adsorbent surfaces; adsorbents’ chemical structures; adsorption capacities; biosorption; elements’ removal efficiency; environmental remediation; food waste adsorbents; heavy metal wastewater; low-cost materials; metals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Hegazi H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013;9:276–282. doi: 10.1016/j.hbrcj.2013.08.004. - DOI
-
- Owlad M., Aroua M.K., Daud W.A.W., Baroutian S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009;200:59–77. doi: 10.1007/s11270-008-9893-7. - DOI
-
- Kamar F.H., Craciun M.E., Nechifor A.C. Heavy metals: Sources, health effects, environmental effects, removal methods and natural adsorbent material as low-cost adsorbent: Short review. Int. J. Sci. Technol. Res. 2014;3:2974–2979.
-
- Aggarwal D., Goyal M., Bansal R.C. Adsorption of chromium by activated carbon from aqueous solution. Carbon. 1999;37:1989–1997. doi: 10.1016/S0008-6223(99)00072-X. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

