Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests
- PMID: 29495424
- PMCID: PMC5872065
- DOI: 10.3390/bios8010017
Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests
Abstract
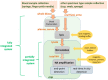
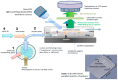
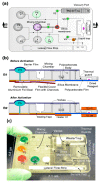
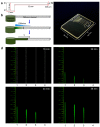
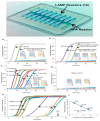
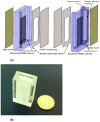
Designs and applications of microfluidics-based devices for molecular diagnostics (Nucleic Acid Amplification Tests, NAATs) in infectious disease testing are reviewed, with emphasis on minimally instrumented, point-of-care (POC) tests for resource-limited settings. Microfluidic cartridges ('chips') that combine solid-phase nucleic acid extraction; isothermal enzymatic nucleic acid amplification; pre-stored, paraffin-encapsulated lyophilized reagents; and real-time or endpoint optical detection are described. These chips can be used with a companion module for separating plasma from blood through a combined sedimentation-filtration effect. Three reporter types: Fluorescence, colorimetric dyes, and bioluminescence; and a new paradigm for end-point detection based on a diffusion-reaction column are compared. Multiplexing (parallel amplification and detection of multiple targets) is demonstrated. Low-cost detection and added functionality (data analysis, control, communication) can be realized using a cellphone platform with the chip. Some related and similar-purposed approaches by others are surveyed.
Keywords: LAMP (loop mediated amplification); NAAT; RPA; lab on a chip; microfluidics; molecular diagnostics; nucleic acid amplification test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

