The Pharmaceutical Industry in 2017. An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 29495494
- PMCID: PMC6017390
- DOI: 10.3390/molecules23030533
The Pharmaceutical Industry in 2017. An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
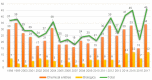
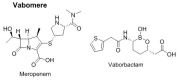
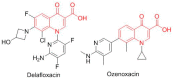
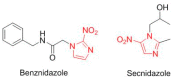
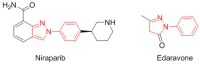
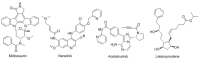
This is an analysis from a chemical point of view of the 46 drugs (34 New Chemical Entities and 12 Biologics) approved by the FDA during 2017. The drugs included in the 2017 "harvest" have been classified on the basis of their chemical structure: biologics (antibodies and proteins); peptides; amino acids and natural products; drug combinations; and small molecules.
Keywords: API; biologics; chemical entities; drug discovery; peptide; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

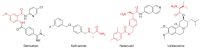





References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 27 February 2018)]; Available online: http://www.accessdata.fda.gov/scripts/cder/daf/
-
- Jarvis L.M. The year in new drugs. Chem. Eng. News. 2018;January 22:26–30.
-
- U.S. Food and Drug Administration (FDA) [(accessed on 27 February 2018)]; Available online: Https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTo....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

