Prorocentrolide-A from Cultured Prorocentrum lima Dinoflagellates Collected in Japan Blocks Sub-Types of Nicotinic Acetylcholine Receptors
- PMID: 29495549
- PMCID: PMC5869385
- DOI: 10.3390/toxins10030097
Prorocentrolide-A from Cultured Prorocentrum lima Dinoflagellates Collected in Japan Blocks Sub-Types of Nicotinic Acetylcholine Receptors
Abstract
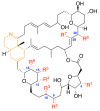
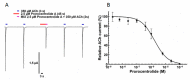
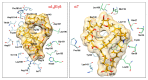
Prorocentrolides are members of the cyclic imine phycotoxins family. Their chemical structure includes a 26-membered carbo-macrocycle and a 28-membered macrocyclic lactone arranged around a hexahydroisoquinoline that incorporates the characteristic cyclic imine group. Six prorocentrolides are already known. However, their mode of action remains undetermined. The aim of the present work was to explore whether prorocentrolide A acts on nicotinic acetylcholine receptors (nAChRs), using competition-binding assays and electrophysiological techniques. Prorocentrolide-A displaced [125I]α-bungarotoxin binding to Torpedo membranes, expressing the muscle-type (α1₂β1γδ) nAChR, and in HEK-293 cells, expressing the chimeric chick neuronal α7-5HT₃ nAChR. Functional studies revealed that prorocentrolide-A had no agonist action on nAChRs, but inhibited ACh-induced currents in Xenopus oocytes that had incorporated the muscle-type α1₂β1γδ nAChR to their membranes, or that expressed the human α7 nAChR, as revealed by voltage-clamp recordings. Molecular docking calculations showed the absence of the characteristic hydrogen bond between the iminium group of prorocentrolide-A and the backbone carbonyl group of Trp147 in the receptor, explaining its weaker affinity as compared to all other cyclic imine toxins. In conclusion, this is the first study to show that prorocentrolide-A acts on both muscle and neuronal nAChRs, but with higher affinity on the muscle-type nAChR.
Keywords: Xenopus oocytes; binding assays; cyclic imine toxins; dinoflagellate toxin; molecular docking; nicotinic acetylcholine receptors; nicotinic currents; prorocentrolides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hoppenrath M., Chomérat N., Horiguchi T., Schweikert M., Nagahama Y., Murray S. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—A proposal and review. Harmful Algae. 2013;27:1–28. doi: 10.1016/j.hal.2013.03.006. - DOI
-
- Cembella A.D. Occurrence of okadaic acid, a major diarrheic shellfish toxin, in natural populations of Dinophysis spp. from the eastern coast of North America. J. Appl. Phycol. 1989;1:307–310. doi: 10.1007/BF00003466. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

