Biochemical and MALDI-TOF Mass Spectrometric Characterization of a Novel Native and Recombinant Cystine Knot Miniprotein from Solanum tuberosum subsp. andigenum cv. Churqueña
- PMID: 29495576
- PMCID: PMC5877539
- DOI: 10.3390/ijms19030678
Biochemical and MALDI-TOF Mass Spectrometric Characterization of a Novel Native and Recombinant Cystine Knot Miniprotein from Solanum tuberosum subsp. andigenum cv. Churqueña
Abstract
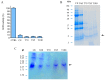
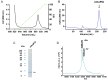
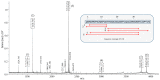
Cystine-knot miniproteins (CKMPs) are an intriguing group of cysteine-rich molecules that combine the characteristics of proteins and peptides. Typically, CKMPs are fewer than 50 residues in length and share a characteristic knotted scaffold characterized by the presence of three intramolecular disulfide bonds that form the singular knotted structure. The knot scaffold confers on these proteins remarkable chemical, thermal, and proteolytic stability. Recently, CKMPs have emerged as a novel class of natural molecules with interesting pharmacological properties. In the present work, a novel cystine-knot metallocarboxypeptidase inhibitor (chuPCI) was isolated from tubers of Solanum tuberosum, subsp. andigenum cv. Churqueña. Our results demonstrated that chuPCI is a member of the A/B-type family of metallocarboxypeptidases inhibitors. chuPCI was expressed and characterized by a combination of biochemical and mass spectrometric techniques. Direct comparison of the MALDI-TOF mass spectra for the native and recombinant molecules allowed us to confirm the presence of four different forms of chuPCI in the tubers. The majority of such forms have a molecular weight of 4309 Da and contain a cyclized Gln in the N-terminus. The other three forms are derived from N-terminal and/or C-terminal proteolytic cleavages. Taken together, our results contribute to increase the current repertoire of natural CKMPs.
Keywords: Andean potatoes; Solanum tuberosum; carboxypeptidase inhibitor; cystine-knot miniproteins; plant inhibitor; protease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Biochemical characterization of a novel carboxypeptidase inhibitor from a variety of Andean potatoes.Phytochemistry. 2015 Dec;120:36-45. doi: 10.1016/j.phytochem.2015.09.010. Epub 2015 Oct 28. Phytochemistry. 2015. PMID: 26521146
-
Biochemical characterization of the YBPCI miniprotein, the first carboxypeptidase inhibitor isolated from Yellow Bell Pepper (Capsicum annuum L). A novel contribution to the knowledge of miniproteins stability.Protein Expr Purif. 2018 Apr;144:55-61. doi: 10.1016/j.pep.2017.12.003. Epub 2017 Dec 6. Protein Expr Purif. 2018. PMID: 29223927
-
Characterization of the wound-induced metallocarboxypeptidase inhibitor from potato. cDNA sequence, induction of gene expression, subcellular immunolocalization and potential roles of the C-terminal propeptide.FEBS Lett. 1998 Nov 27;440(1-2):175-82. doi: 10.1016/s0014-5793(98)01447-1. FEBS Lett. 1998. PMID: 9862450
-
Natural and engineered cystine knot miniproteins for diagnostic and therapeutic applications.Curr Pharm Des. 2011 Dec;17(38):4329-36. doi: 10.2174/138161211798999465. Curr Pharm Des. 2011. PMID: 22204431 Review.
-
Synthetic Cystine-Knot Miniproteins - Valuable Scaffolds for Polypeptide Engineering.Adv Exp Med Biol. 2016;917:121-44. doi: 10.1007/978-3-319-32805-8_7. Adv Exp Med Biol. 2016. PMID: 27236555 Review.
Cited by
-
Molecular Insights into the Role of Cysteine-Rich Peptides in Induced Resistance to Fusarium oxysporum Infection in Tomato Based on Transcriptome Profiling.Int J Mol Sci. 2021 May 27;22(11):5741. doi: 10.3390/ijms22115741. Int J Mol Sci. 2021. PMID: 34072144 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

