Ion Channel Disorders and Sudden Cardiac Death
- PMID: 29495624
- PMCID: PMC5877553
- DOI: 10.3390/ijms19030692
Ion Channel Disorders and Sudden Cardiac Death
Abstract
Long QT syndrome, short QT syndrome, Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia are inherited primary electrical disorders that predispose to sudden cardiac death in the absence of structural heart disease. Also known as cardiac channelopathies, primary electrical disorders respond to mutations in genes encoding cardiac ion channels and/or their regulatory proteins, which result in modifications in the cardiac action potential or in the intracellular calcium handling that lead to electrical instability and life-threatening ventricular arrhythmias. These disorders may have low penetrance and expressivity, making clinical diagnosis often challenging. However, because sudden cardiac death might be the first presenting symptom of the disease, early diagnosis becomes essential. Genetic testing might be helpful in this regard, providing a definite diagnosis in some patients. Yet important limitations still exist, with a significant proportion of patients remaining with no causative mutation identifiable after genetic testing. This review aims to provide the latest knowledge on the genetic basis of cardiac channelopathies and discuss the role of the affected proteins in the pathophysiology of each one of these diseases.
Keywords: Brugada syndrome; catecholaminergic polymorphic ventricular tachycardia; channelopathies; ion channel; long QT syndrome; primary electrical disorders; short QT syndrome; sudden cardiac death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
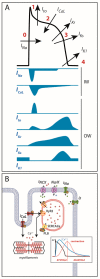
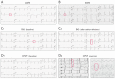
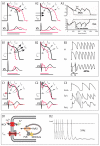
References
-
- Fishman G.I., Chugh S., DiMarco J., Albert C., Anderson M., Bonow R., Buxton A., Chen P.-S., Estes M., Jouven X., et al. Sudden Cardiac Death Prediction and Prevention Report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. - DOI - PMC - PubMed
-
- Eckart R.E., Shry E.A., Burke A.P., McNear J.A., Appel D.A., Castillo-Rojas L.M., Avedissian L., Pearse L.A., Potter R.N., Tremaine L., et al. Sudden death in young adults: An autopsy-based series of a population undergoing active surveillance. J. Am. Coll. Cardiol. 2011;58:1254–1261. doi: 10.1016/j.jacc.2011.01.049. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

