A Finite Element Solution of Lateral Periodic Poisson-Boltzmann Model for Membrane Channel Proteins
- PMID: 29495644
- PMCID: PMC5877556
- DOI: 10.3390/ijms19030695
A Finite Element Solution of Lateral Periodic Poisson-Boltzmann Model for Membrane Channel Proteins
Abstract
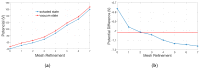
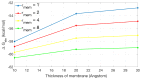
Membrane channel proteins control the diffusion of ions across biological membranes. They are closely related to the processes of various organizational mechanisms, such as: cardiac impulse, muscle contraction and hormone secretion. Introducing a membrane region into implicit solvation models extends the ability of the Poisson-Boltzmann (PB) equation to handle membrane proteins. The use of lateral periodic boundary conditions can properly simulate the discrete distribution of membrane proteins on the membrane plane and avoid boundary effects, which are caused by the finite box size in the traditional PB calculations. In this work, we: (1) develop a first finite element solver (FEPB) to solve the PB equation with a two-dimensional periodicity for membrane channel proteins, with different numerical treatments of the singular charges distributions in the channel protein; (2) add the membrane as a dielectric slab in the PB model, and use an improved mesh construction method to automatically identify the membrane channel/pore region even with a tilt angle relative to the z-axis; and (3) add a non-polar solvation energy term to complete the estimation of the total solvation energy of a membrane protein. A mesh resolution of about 0.25 Å (cubic grid space)/0.36 Å (tetrahedron edge length) is found to be most accurate in linear finite element calculation of the PB solvation energy. Computational studies are performed on a few exemplary molecules. The results indicate that all factors, the membrane thickness, the length of periodic box, membrane dielectric constant, pore region dielectric constant, and ionic strength, have individually considerable influence on the solvation energy of a channel protein. This demonstrates the necessity to treat all of those effects in the PB model for membrane protein simulations.
Keywords: finite element method; laterally periodic Poisson-Boltzmann model; membrane channel proteins; pore region; solvation.
Conflict of interest statement
The authors declare no conflict of interests.
Figures










References
-
- Honig B., Sharp K., Yang A.S. Macroscopic models of aqueous solutions: Biological and chemical applications. J. Phys. Chem. 1993;97:1101–1109. doi: 10.1021/j100108a002. - DOI
-
- Beglov D., Roux B. Solvation of complex molecules in a polar liquid: An integral equation theory. J. Chem. Phys. 1996;104:8678–8689. doi: 10.1063/1.471557. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

