Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants
- PMID: 29496172
- PMCID: PMC5947877
- DOI: 10.1016/j.phymed.2017.12.032
Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants
Abstract
Background: Monoamine oxidases (MAOs) are outer mitochondrial membrane flavoenzymes. They catalyze the oxidative deamination of a variety of neurotransmitters. MAO-A and MAO-B may be considered as targets for inhibitors to treat neurodegenerative diseases and depression and for managing symptoms associated with Parkinson's and Alzheimer's diseases.
Purpose: The objective was to evaluate the inhibitory effect of Hypericum afrum and Cytisus villosus against MAO-A and B and to isolate the compounds responsible for the MAO-inhibitory activity.
Methods: The inhibitory effect of extracts and purified constituents of H. afrum and C. villosus were investigated in vitro using recombinant human MAO-A and B, and through bioassay-guided fractionation of ethyl acetate fractions of areal parts of the two plants collected in northeastern Algeria. In addition, computational protein-ligand docking and molecular dynamics simulations were carried out to explain the MAO binding at the molecular level.
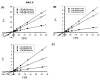
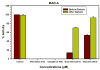
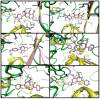
Results: The ethyl acetate (EtOAc) fractions of H. afrum and C. villosus showed the highest MAO inhibition activity against MAO A and B with IC50 values of 3.37 µg/ml and 13.50 µg/ml as well as 5.62 and 1.87 µg/ml, respectively. Bioassay-guided fractionation of the EtOAc fractions resulted in the purification and identification of the known flavonoids quercetin, myricetin, genistein and chrysin as the principal MAO-inhibitory constituents. Their structures were established by extensive 1 and 2D NMR studies and mass spectrometry. Quercetin, myricetin and chrysin showed potent inhibitory activity towards MAO-A with IC50 values of 1.52, 9.93 and 0.25 µM, respectively, while genistein more efficiently inhibited MAO-B (IC50 value: 0.65 µM). The kinetics of the inhibition and the study of dialysis dissociation of the complex of quercetin and myricetin and the isoenzyme MAO-A showed competitive and mixed inhibition, respectively. Both compounds showed reversible binding. Molecular docking experiments and molecular dynamics simulations allowed to estimate the binding poses and to identify the most important residues involved in the selective recognition of molecules in the MAOs enzymatic clefts.
Conclusion: Quercetin and myricetin isolated from H. afrum together with genistein and chrysin isolated from C. villosus have been identified as potent MAO-A and -B inhibitors. H. afrum and C. villosus have properties indicative of potential neuroprotective ability and may be new candidates for selective MAO-A and B inhibitors.
Keywords: Antidepressant; Bioassay-guided fractionation; HR-ESI-MS; Molecular docking; Monoamine oxidase; NMR.
Copyright © 2017 Elsevier GmbH. All rights reserved.
Conflict of interest statement
The authors declare there are no conflict of interest.
Figures
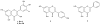

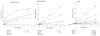

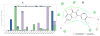

Similar articles
-
Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study.J Nat Prod. 2006 Jun;69(6):945-9. doi: 10.1021/np060015w. J Nat Prod. 2006. PMID: 16792415
-
The Benzopyrone Biochanin-A as a reversible, competitive, and selective monoamine oxidase B inhibitor.BMC Complement Altern Med. 2017 Jan 10;17(1):34. doi: 10.1186/s12906-016-1525-y. BMC Complement Altern Med. 2017. PMID: 28069007 Free PMC article.
-
Proposed Mechanism for the Antitrypanosomal Activity of Quercetin and Myricetin Isolated from Hypericum afrum Lam.: Phytochemistry, In Vitro Testing and Modeling Studies.Molecules. 2021 Feb 14;26(4):1009. doi: 10.3390/molecules26041009. Molecules. 2021. PMID: 33672916 Free PMC article.
-
Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders.Molecules. 2019 Jan 24;24(3):418. doi: 10.3390/molecules24030418. Molecules. 2019. PMID: 30678358 Free PMC article. Review.
-
Selective MAO-B inhibitors: a lesson from natural products.Mol Divers. 2014 Feb;18(1):219-43. doi: 10.1007/s11030-013-9490-6. Epub 2013 Nov 12. Mol Divers. 2014. PMID: 24218136 Review.
Cited by
-
Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities.Int J Environ Res Public Health. 2022 Sep 30;19(19):12495. doi: 10.3390/ijerph191912495. Int J Environ Res Public Health. 2022. PMID: 36231792 Free PMC article. Review.
-
Mechanistic Understanding from Molecular Dynamics in Pharmaceutical Research 2: Lipid Membrane in Drug Design.Pharmaceuticals (Basel). 2021 Oct 19;14(10):1062. doi: 10.3390/ph14101062. Pharmaceuticals (Basel). 2021. PMID: 34681286 Free PMC article. Review.
-
Untargeted Metabolomics Analysis of the Orchid Species Oncidium sotoanum Reveals the Presence of Rare Bioactive C-Diglycosylated Chrysin Derivatives.Plants (Basel). 2023 Feb 2;12(3):655. doi: 10.3390/plants12030655. Plants (Basel). 2023. PMID: 36771739 Free PMC article.
-
Chemical Constituents and Antidepressant-Like Effects in Ovariectomized Mice of the Ethanol Extract of Alternanthera philoxeroides.Molecules. 2018 Aug 31;23(9):2202. doi: 10.3390/molecules23092202. Molecules. 2018. PMID: 30200295 Free PMC article.
-
Multiscale Simulations of Biological Membranes: The Challenge To Understand Biological Phenomena in a Living Substance.Chem Rev. 2019 May 8;119(9):5607-5774. doi: 10.1021/acs.chemrev.8b00538. Epub 2019 Mar 12. Chem Rev. 2019. PMID: 30859819 Free PMC article.
References
-
- Desmond Molecular Dynamics System, version 4.4. D. E. Shaw Research; New York, NY: 2015.
-
- Maestro-Desmond Interoperability Tools, version 4.4. Schrödinger; New York, NY: 2015.
-
- Epik, version 3.4. Schrödinger, LLC; New York, NY: 2015.
-
- Glide, version 6.9. Schrödinger, LLC; New York, NY: 2015.
-
- LigPrep, version 3.6. Schrödinger, LLC; New York, NY: 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

