Optogenetic fMRI and electrophysiological identification of region-specific connectivity between the cerebellar cortex and forebrain
- PMID: 29496611
- PMCID: PMC5911204
- DOI: 10.1016/j.neuroimage.2018.02.047
Optogenetic fMRI and electrophysiological identification of region-specific connectivity between the cerebellar cortex and forebrain
Abstract
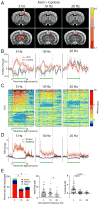
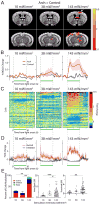
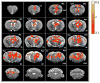
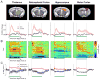
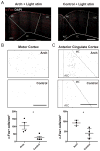
Complex animal behavior is produced by dynamic interactions between discrete regions of the brain. As such, defining functional connections between brain regions is critical in gaining a full understanding of how the brain generates behavior. Evidence suggests that discrete regions of the cerebellar cortex functionally project to the forebrain, mediating long-range communication potentially important in motor and non-motor behaviors. However, the connectivity map remains largely incomplete owing to the challenge of driving both reliable and selective output from the cerebellar cortex, as well as the need for methods to detect region specific activation across the entire forebrain. Here we utilize a paired optogenetic and fMRI (ofMRI) approach to elucidate the downstream forebrain regions modulated by activating a region of the cerebellum that induces stereotypical, ipsilateral forelimb movements. We demonstrate with ofMRI, that activating this forelimb motor region of the cerebellar cortex results in functional activation of a variety of forebrain and midbrain areas of the brain, including the hippocampus and primary motor, retrosplenial and anterior cingulate cortices. We further validate these findings using optogenetic stimulation paired with multi-electrode array recordings and post-hoc staining for molecular markers of activated neurons (i.e. c-Fos). Together, these findings demonstrate that a single discrete region of the cerebellar cortex is capable of influencing motor output and the activity of a number of downstream forebrain as well as midbrain regions thought to be involved in different aspects of behavior.
Keywords: Cerebellum; Hippocampus; Motor cortex; Prefrontal cortex; Thalamus; ofMRI.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

References
-
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Marien P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J. Consensus Paper: Cerebellum and Emotion. Cerebellum. 2017;16:552–576. http://doi.org/10.1007/s12311-016-0815-8. - DOI - PubMed
-
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M. High field BOLD response to forepaw stimulation in the mouse. Neuroimage. 2010;51:704–712. http://doi.org/10.1016/j.neuroimage.2010.02.083. - DOI - PubMed
-
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. http://doi.org/10.1523/jneurosci.3134-07.2007. - DOI - PMC - PubMed
-
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54:957–1006. - PubMed
-
- Amianto F, D’Agata F, Lavagnino L, Caroppo P, Abbate-Daga G, Righi D, Scarone S, Bergui M, Mortara P, Fassino S. Intrinsic Connectivity Networks Within Cerebellum and Beyond in Eating Disorders. The Cerebellum. 2013;12:623–631. http://doi.org/10.1007/s12311-013-0471-1. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

