Ly6CHi Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus
- PMID: 29496661
- PMCID: PMC5920725
- DOI: 10.1161/ATVBAHA.118.310703
Ly6CHi Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus
Abstract
Objective: Wound monocyte-derived macrophage plasticity controls the initiation and resolution of inflammation that is critical for proper healing, however, in diabetes mellitus, the resolution of inflammation fails to occur. In diabetic wounds, the kinetics of blood monocyte recruitment and the mechanisms that control in vivo monocyte/macrophage differentiation remain unknown.
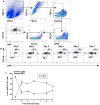
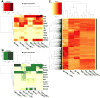
Approach and results: Here, we characterized the kinetics and function of Ly6CHi [Lin- (CD3-CD19-NK1.1-Ter-119-) Ly6G-CD11b+] and Ly6CLo [Lin- (CD3-CD19-NK1.1-Ter-119-) Ly6G-CD11b+] monocyte/macrophage subsets in normal and diabetic wounds. Using flow-sorted tdTomato-labeled Ly6CHi monocyte/macrophages, we show Ly6CHi cells transition to a Ly6CLo phenotype in normal wounds, whereas in diabetic wounds, there is a late, second influx of Ly6CHi cells that fail transition to Ly6CLo. The second wave of Ly6CHi cells in diabetic wounds corresponded to a spike in MCP-1 (monocyte chemoattractant protein-1) and selective administration of anti-MCP-1 reversed the second Ly6CHi influx and improved wound healing. To examine the in vivo phenotype of wound monocyte/macrophages, RNA-seq-based transcriptome profiling was performed on flow-sorted Ly6CHi [Lin-Ly6G-CD11b+] and Ly6CLo [Lin-Ly6G-CD11b+] cells from normal and diabetic wounds. Gene transcriptome profiling of diabetic wound Ly6CHi cells demonstrated differences in proinflammatory and profibrotic genes compared with controls.
Conclusions: Collectively, these data identify kinetic and functional differences in diabetic wound monocyte/macrophages and demonstrate that selective targeting of CD11b+Ly6CHi monocyte/macrophages is a viable therapeutic strategy for inflammation in diabetic wounds.
Keywords: diabetes mellitus; inflammation; macrophages; monocytes; wound healing.
© 2018 American Heart Association, Inc.
Conflict of interest statement
Conflict of Interest Statement: The authors have no conflict of interest to declare.
Figures

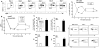
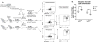
References
-
- Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, Boulton AJ. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22:157–62. - PubMed
-
- Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5-year follow-up. Diabetes Care. 2001;24:78–83. - PubMed
-
- Kannel WB. Risk factors for atherosclerotic cardiovascular outcomes in different arterial territories. J Cardiovasc Risk. 1994;1:333–9. - PubMed
-
- Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

