Myosin-Driven Intracellular Transport
- PMID: 29496823
- PMCID: PMC5830894
- DOI: 10.1101/cshperspect.a021972
Myosin-Driven Intracellular Transport
Abstract
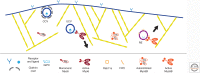
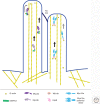
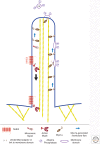
The delivery of intracellular material within cells is crucial for maintaining normal function. Myosins transport a wide variety of cargo, ranging from vesicles to ribonuclear protein particles (RNPs), in plants, fungi, and metazoa. The properties of a given myosin transporter are adapted to move on different actin filament tracks, either on the disordered actin networks at the cell cortex or along highly organized actin bundles to distribute their cargo in a localized manner or move it across long distances in the cell. Transport is controlled by selective recruitment of the myosin to its cargo that also plays a role in activation of the motor.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Ameen N, Apodaca G. 2007. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
