Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions
- PMID: 29496890
- PMCID: PMC5833009
- DOI: 10.1124/pr.117.014647
Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions
Abstract
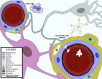
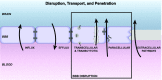
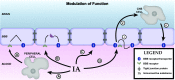
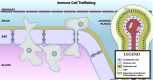
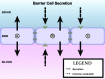
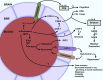
Central nervous system (CNS) barriers predominantly mediate the immune-privileged status of the brain, and are also important regulators of neuroimmune communication. It is increasingly appreciated that communication between the brain and immune system contributes to physiologic processes, adaptive responses, and disease states. In this review, we discuss the highly specialized features of brain barriers that regulate neuroimmune communication in health and disease. In section I, we discuss the concept of immune privilege, provide working definitions of brain barriers, and outline the historical work that contributed to the understanding of CNS barrier functions. In section II, we discuss the unique anatomic, cellular, and molecular characteristics of the vascular blood-brain barrier (BBB), blood-cerebrospinal fluid barrier, and tanycytic barriers that confer their functions as neuroimmune interfaces. In section III, we consider BBB-mediated neuroimmune functions and interactions categorized as five neuroimmune axes: disruption, responses to immune stimuli, uptake and transport of immunoactive substances, immune cell trafficking, and secretions of immunoactive substances. In section IV, we discuss neuroimmune functions of CNS barriers in physiologic and disease states, as well as pharmacological interventions for CNS diseases. Throughout this review, we highlight many recent advances that have contributed to the modern understanding of CNS barriers and their interface functions.
Copyright © 2018 by The Author(s).
Figures

References
-
- Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. (2015) Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur J Immunol 45:1043–1058. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

