A potent complement factor C3-specific nanobody inhibiting multiple functions in the alternative pathway of human and murine complement
- PMID: 29497000
- PMCID: PMC5925797
- DOI: 10.1074/jbc.RA117.001179
A potent complement factor C3-specific nanobody inhibiting multiple functions in the alternative pathway of human and murine complement
Erratum in
-
Correction: A potent complement factor C3-specific nanobody inhibiting multiple functions in the alternative pathway of human and murine complement.J Biol Chem. 2023 Feb;299(2):102951. doi: 10.1016/j.jbc.2023.102951. Epub 2023 Feb 4. J Biol Chem. 2023. PMID: 36739657 Free PMC article. No abstract available.
Abstract
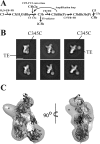
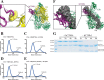
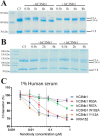
The complement system is a complex, carefully regulated proteolytic cascade for which suppression of aberrant activation is of increasing clinical relevance, and inhibition of the complement alternative pathway is a subject of intense research. Here, we describe the nanobody hC3Nb1 that binds to multiple functional states of C3 with subnanomolar affinity. The nanobody causes a complete shutdown of alternative pathway activity in human and murine serum when present in concentrations comparable with that of C3, and hC3Nb1 is shown to prevent proconvertase assembly, as well as binding of the C3 substrate to C3 convertases. Our crystal structure of the C3b-hC3Nb1 complex and functional experiments demonstrate that proconvertase formation is blocked by steric hindrance between the nanobody and an Asn-linked glycan on complement factor B. In addition, hC3Nb1 is shown to prevent factor H binding to C3b, rationalizing its inhibition of factor I activity. Our results identify hC3Nb1 as a versatile, inexpensive, and powerful inhibitor of the alternative pathway in both human and murine in vitro model systems of complement activation.
Keywords: alternative pathway; antibody; complement; convertase; inhibitor; innate immunity; structural biology.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Fishelson Z., and Müller-Eberhard H. J. (1982) C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J. Immunol. 129, 2603–2607 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

