The Complement System Is Critical in Maintaining Retinal Integrity during Aging
- PMID: 29497373
- PMCID: PMC5818470
- DOI: 10.3389/fnagi.2018.00015
The Complement System Is Critical in Maintaining Retinal Integrity during Aging
Abstract
The complement system is a key component of innate immunity comprised of soluble components that form a proteolytic cascade leading to the generation of effector molecules involved in cellular clearance. This system is highly activated not only under general inflammatory conditions such as infections, collagen diseases, nephritis, and liver diseases, but also in focal ocular diseases. However, little is known about the role of the complement system in retinal homeostasis during aging. Using young (6-week-old) and adult (6-month-old) mice in wild type (C57BL/6) and complement knockout strains (C1q-/-, Mbl a/c-/-, Fb-/-, C3-/-, and C5-/-), we compared amplitudes of electroretinograms (ERG) and thicknesses of retinal layers in spectral domain optical coherence tomography between young and adult mice. The ERG amplitudes in adult mice were significantly decreased (p < 0.001, p < 0.0001) compared to that of young mice in all complement knockout strains, and there were significant decreases in the inner nuclear layer (INL) thickness in adult mice compared to young mice in all complement knockout strains (p < 0.0001). There were no significant differences in ERG amplitude or thickness of the INL between young and adult control mice. These data suggest that the complement system plays an important role in maintaining normal retinal integrity over time.
Keywords: EM; ERG; OCT; aging; complement system; retina.
Figures

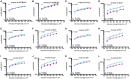

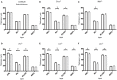





References
-
- Anderson D. H., Radeke M. J., Gallo N. B., Chapin E. A., Johnson P. T., Curletti C. R., et al. . (2010). The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res. 29, 95–112. 10.1016/j.preteyeres.2009.11.003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

