African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System
- PMID: 29497418
- PMCID: PMC5818406
- DOI: 10.3389/fimmu.2018.00218
African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System
Abstract
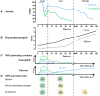
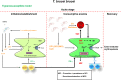
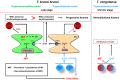
African trypanosomosis (AT) is a chronically debilitating parasitic disease of medical and economic importance for the development of sub-Saharan Africa. The trypanosomes that cause this disease are extracellular protozoan parasites that have developed efficient immune escape mechanisms to manipulate the entire host immune response to allow parasite survival and transmission. During the early stage of infection, a profound pro-inflammatory type 1 activation of the mononuclear phagocyte system (MPS), involving classically activated macrophages (i.e., M1), is required for initial parasite control. Yet, the persistence of this M1-type MPS activation in trypanosusceptible animals causes immunopathology with anemia as the most prominent pathological feature. By contrast, in trypanotolerant animals, there is an induction of IL-10 that promotes the induction of alternatively activated macrophages (M2) and collectively dampens tissue damage. A comparative gene expression analysis between M1 and M2 cells identified galectin-3 (Gal-3) and macrophage migration inhibitory factor (MIF) as novel M1-promoting factors, possibly acting synergistically and in concert with TNF-α during anemia development. While Gal-3 enhances erythrophagocytosis, MIF promotes both myeloid cell recruitment and iron retention within the MPS, thereby depriving iron for erythropoiesis. Hence, the enhanced erythrophagocytosis and suppressed erythropoiesis lead to anemia. Moreover, a thorough investigation using MIF-deficient mice revealed that the underlying mechanisms in AT-associated anemia development in trypanosusceptible and tolerant animals are quite distinct. In trypanosusceptible animals, anemia resembles anemia of inflammation, while in trypanotolerant animals' hemodilution, mainly caused by hepatosplenomegaly, is an additional factor contributing to anemia. In this review, we give an overview of how trypanosome- and host-derived factors can contribute to trypanosomosis-associated anemia development with a focus on the MPS system. Finally, we will discuss potential intervention strategies to alleviate AT-associated anemia that might also have therapeutic potential.
Keywords: IFN-γ; IL-10; MIF; MPS; anemia; erythrophagocytosis; hemodilution; inflammation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

