Engineered T Regulatory Type 1 Cells for Clinical Application
- PMID: 29497421
- PMCID: PMC5818395
- DOI: 10.3389/fimmu.2018.00233
Engineered T Regulatory Type 1 Cells for Clinical Application
Abstract
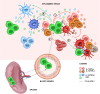
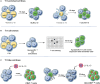
T regulatory cells, a specialized subset of T cells, are key players in modulating antigen (Ag)-specific immune responses in vivo. Inducible T regulatory type 1 (Tr1) cells are characterized by the co-expression of CD49b and lymphocyte-activation gene 3 (LAG-3) and the ability to secrete IL-10, TGF-β, and granzyme (Gz) B, in the absence of IL-4 and IL-17. The chief mechanisms by which Tr1 cells control immune responses are secretion of IL-10 and TGF-β and killing of myeloid cells via GzB. Tr1 cells, first described in peripheral blood of patients who developed tolerance after HLA-mismatched fetal liver hematopoietic stem cell transplantation, have been proven to modulate inflammatory and effector T cell responses in several immune-mediated diseases. The possibility to generate and expand Tr1 cells in vitro in an Ag-specific manner has led to their clinical use as cell therapy in patients. Clinical grade protocols to generate or to enrich and expand Tr1 cell medicinal products have been established. Proof-of-concept clinical trials with Tr1 cell products have demonstrated the safety and the feasibility of this approach and indicated some clinical benefit. In the present review, we provide an overview on protocols established to induce/expand Tr1 cells in vitro for clinical application and on results obtained in Tr1 cell-based clinical trials. Moreover, we will discuss a recently developed protocol to efficient convert human CD4+ T cells into a homogeneous population of Tr1-like cells by lentiviral vector-mediated IL-10 gene transfer.
Keywords: IL-10; T regulatory cell-based therapy; T regulatory type 1 (Tr1) cells; gene transfer; tolerance.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

