Performance Survey and Comparison Between Rapid Sterility Testing Method and Pharmacopoeia Sterility Test
- PMID: 29497461
- PMCID: PMC5816116
- DOI: 10.1007/s12247-017-9303-z
Performance Survey and Comparison Between Rapid Sterility Testing Method and Pharmacopoeia Sterility Test
Abstract
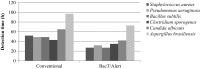
The sterility test described in pharmacopoeial compendia requires a 14-day incubation period to obtain a valid analytical result. Therefore, the use of alternative methods to evaluate the sterility of pharmaceuticals, such as the BacT/Alert® 3D system, is particularly interesting, because it allows a reduced incubation period and lower associated costs. Considering that the BacT/Alert® 3D system offers several culture media formulations developed for this microbial detection system, the present study was aimed to evaluate and compare the performance of BacT/Alert® 3D with the pharmacopoeial sterility test. There was no significant difference between the ability of the culture media to allow detection of microbial contamination. However, the rapid sterility testing method allowed a more rapid detection of the challenge microorganisms, which indicates that the system is a viable alternative for assessing the sterility of injectable products.
Keywords: Culture media; Rapid microbiological method; Sterility test; Sterility testing performance.
Figures
References
-
- Sandle T. Sterility test failure investigations. J GXP Compliance 2012;16(1):1–10.
-
- USP. Validation of alternative microbiological methods. In: United States Pharmacopeia. 40th ed. Rockville: The United States Pharmacopeial Convention, Inc; 2017.
-
- Cundel A. Opportunities for rapid microbial methods. American Pharmaceutical Review. December 2006: http://www.americanpharmaceuticalreview.com/FeaturedArticles/113427-Oppo....
-
- Gray JC, Stärk A, Berchtold M, Mercier M, Neuhaus G, Wirth A. Introduction of a rapid microbiological method as an alternative to the pharmacopoeial method for the sterility test. Am Pharmaceut Rev. 2010;13(6):88–94.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases