Piezoelectric smart biomaterials for bone and cartilage tissue engineering
- PMID: 29497465
- PMCID: PMC5828134
- DOI: 10.1186/s41232-018-0059-8
Piezoelectric smart biomaterials for bone and cartilage tissue engineering
Abstract
Tissues like bone and cartilage are remodeled dynamically for their functional requirements by signaling pathways. The signals are controlled by the cells and extracellular matrix and transmitted through an electrical and chemical synapse. Scaffold-based tissue engineering therapies largely disturb the natural signaling pathways, due to their rigidity towards signal conduction, despite their therapeutic advantages. Thus, there is a high need of smart biomaterials, which can conveniently generate and transfer the bioelectric signals analogous to native tissues for appropriate physiological functions. Piezoelectric materials can generate electrical signals in response to the applied stress. Furthermore, they can stimulate the signaling pathways and thereby enhance the tissue regeneration at the impaired site. The piezoelectric scaffolds can act as sensitive mechanoelectrical transduction systems. Hence, it is applicable to the regions, where mechanical loads are predominant. The present review is mainly concentrated on the mechanism related to the electrical stimulation in a biological system and the different piezoelectric materials suitable for bone and cartilage tissue engineering.
Keywords: Bone; Cartilage; Electroactive scaffolds; Mechanical stimulation; Piezoelectric materials; Piezoelectricity; Tissue regeneration.
Conflict of interest statement
Not applicable.On behalf of all authors, the corresponding author has given the consent for the publication.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
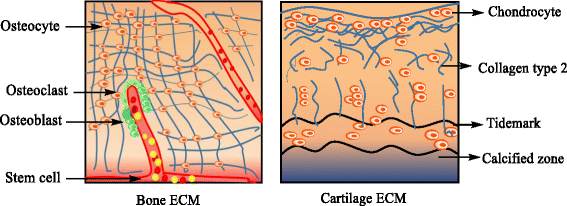

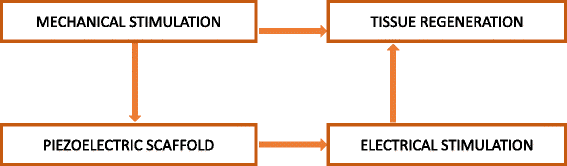
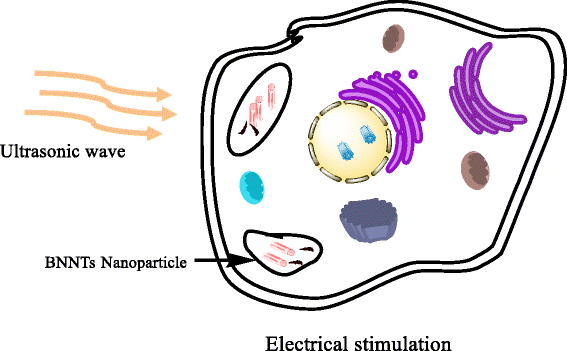
References
-
- Lee SJ, Yoo JJ, Atala A. Biomaterials and tissue engineering. In: Clinical Regenerative Medicine in Urology. Singapore: Springer; 2018. p. 17–51.
-
- Mason WP. Piezoelectricity, its history and applications. The Journal of the Acoustical Society of America. 1981;70(6):1561–1566.
-
- Mindlin R. Elasticity, piezoelectricity and crystal lattice dynamics. J Elast. 1972;2(4):217–282.
-
- Arinzeh T, Collins G, Lee Y-S. Google Patents. 2016. System and method for a piezoelectric scaffold for nerve growth and repair.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

