α-Tubulin Acetyltransferase Is a Novel Target Mediating Neurite Growth Inhibitory Effects of Chondroitin Sulfate Proteoglycans and Myelin-Associated Glycoprotein
- PMID: 29497702
- PMCID: PMC5830348
- DOI: 10.1523/ENEURO.0240-17.2018
α-Tubulin Acetyltransferase Is a Novel Target Mediating Neurite Growth Inhibitory Effects of Chondroitin Sulfate Proteoglycans and Myelin-Associated Glycoprotein
Abstract
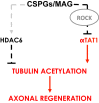
Damage to the CNS results in neuronal and axonal degeneration, and subsequent neurological dysfunction. Endogenous repair in the CNS is impeded by inhibitory chemical and physical barriers, such as chondroitin sulfate proteoglycans (CSPGs) and myelin-associated glycoprotein (MAG), which prevent axon regeneration. Previously, it has been demonstrated that the inhibition of axonal histone deacetylase-6 (HDAC6) can promote microtubule α-tubulin acetylation and restore the growth of CSPGs- and MAG-inhibited axons. Since the acetylation of α-tubulin is regulated by two opposing enzymes, HDAC6 (deacetylation) and α-tubulin acetyltransferase-1 (αTAT1; acetylation), we have investigated the regulation of these enzymes downstream of a growth inhibitory signal. Our findings show that exposure of primary mouse cortical neurons to soluble CSPGs and MAG substrates cause an acute and RhoA-kinase-dependent reduction in α-tubulin acetylation and αTAT1 protein levels, without changes to either HDAC6 levels or HDAC6 activity. The CSPGs- and MAG-induced reduction in αTAT1 occurs primarily in the distal and middle regions of neurites and reconstitution of αTAT1, either by Rho-associated kinase (ROCK) inhibition or lentiviral-mediated αTAT1 overexpression, can restore neurite growth. Lastly, we demonstrate that CSPGs and MAG signaling decreases αTAT1 levels posttranscriptionally via a ROCK-dependent increase in αTAT1 protein turnover. Together, these findings define αTAT1 as a novel potential therapeutic target for ameliorating CNS injury characterized by growth inhibitory substrates that are prohibitive to axonal regeneration.
Keywords: chondroitin sulfate proteoglycan; myelin-associated glycoprotein; α-tubulin acetylation; α-tubulin acetyltransferase.
Figures




References
-
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W (2003) Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 22:405–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
