Genomic Variation and Pharmacokinetics in Old Age: A Quantitative Review of Age- vs. Genotype-Related Differences
- PMID: 29498032
- PMCID: PMC6585622
- DOI: 10.1002/cpt.1057
Genomic Variation and Pharmacokinetics in Old Age: A Quantitative Review of Age- vs. Genotype-Related Differences
Abstract
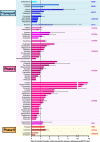
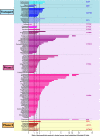
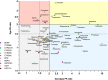
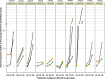
Older persons may particularly benefit from pharmacogenetic diagnostics, but there is little clinical evidence on that question. We quantitatively analyzed the effects of age and genotype in drugs with consensus on a therapeutically relevant impact of a genotype. Assuming additive effects of age and genotype, drugs may be classified in groups with different priorities to consider either age, or genotype, or both, in therapy. Particularly interesting were those studies specifically analyzing the age-by-genotype interaction.
© 2018 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no competing interests for this work.
Figures


Similar articles
-
A genomic "roadmap" to "better" drugs.Drug Metab Rev. 2008;40(2):225-39. doi: 10.1080/03602530801952815. Drug Metab Rev. 2008. PMID: 18464044 Review.
-
Impact of New Genomic Technologies on Understanding Adverse Drug Reactions.Clin Pharmacokinet. 2016 Apr;55(4):419-36. doi: 10.1007/s40262-015-0324-9. Clin Pharmacokinet. 2016. PMID: 26369774 Free PMC article. Review.
-
Use of a multi-gene pharmacogenetic panel reduces adverse drug effects.Cell Rep Med. 2023 May 16;4(5):101021. doi: 10.1016/j.xcrm.2023.101021. Epub 2023 Apr 20. Cell Rep Med. 2023. PMID: 37084734 Free PMC article.
-
Variability in response to medicines in older people: phenotypic and genotypic factors.Clin Pharmacol Ther. 2009 Apr;85(4):431-3. doi: 10.1038/clpt.2009.1. Epub 2009 Feb 18. Clin Pharmacol Ther. 2009. PMID: 19225449 Review. No abstract available.
-
Identifying genomic and developmental causes of adverse drug reactions in children.Pharmacogenomics. 2010 Nov;11(11):1591-602. doi: 10.2217/pgs.10.146. Pharmacogenomics. 2010. PMID: 21121777 Free PMC article. Review.
Cited by
-
Drug-gene interactions in older patients with coronary artery disease.BMC Geriatr. 2024 Oct 26;24(1):881. doi: 10.1186/s12877-024-05471-7. BMC Geriatr. 2024. PMID: 39462319 Free PMC article.
-
Genophenotypic Factors and Pharmacogenomics in Adverse Drug Reactions.Int J Mol Sci. 2021 Dec 10;22(24):13302. doi: 10.3390/ijms222413302. Int J Mol Sci. 2021. PMID: 34948113 Free PMC article. Review.
-
Prescribing medicines to older people-How to consider the impact of ageing on human organ and body functions.Br J Clin Pharmacol. 2020 Oct;86(10):1921-1930. doi: 10.1111/bcp.14094. Epub 2019 Dec 16. Br J Clin Pharmacol. 2020. PMID: 31425638 Free PMC article. Review.
-
Clinical Impact of Implementing a Nurse-Led Adverse Drug Reaction Profile in Older Adults Prescribed Multiple Medicines in UK Primary Care: A Study Protocol for a Cluster-Randomised Controlled Trial.Pharmacy (Basel). 2022 Apr 28;10(3):52. doi: 10.3390/pharmacy10030052. Pharmacy (Basel). 2022. PMID: 35645331 Free PMC article.
-
Individualized versus Standardized Risk Assessment in Patients at High Risk for Adverse Drug Reactions (The IDrug Randomized Controlled Trial)-Never Change a Running System?Pharmaceuticals (Basel). 2021 Oct 18;14(10):1056. doi: 10.3390/ph14101056. Pharmaceuticals (Basel). 2021. PMID: 34681280 Free PMC article.
References
-
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76 (2009). - PubMed
-
- McLean, A.J. & Le Couteur, D.G. Aging biology and geriatric clinical pharmacology. Pharmacol. Rev. 56, 163–184 (2004). - PubMed
-
- Wynne, H.A. , Cope, L.H. , Mutch, E. , Rawlins, M.D. , Woodhouse, K.W. & James, O.F. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 9, 297–301 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

