Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation
- PMID: 29498036
- PMCID: PMC5913400
- DOI: 10.1111/bph.14188
Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation
Abstract
Background and purpose: The mechanosensitive Piezo1 channel has important roles in vascular physiology and disease. Yoda1 is a small-molecule agonist, but the pharmacology of these channels is otherwise limited.
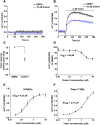
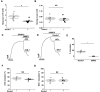
Experimental approach: Yoda1 analogues were generated by synthetic chemistry. Intracellular Ca2+ and Tl+ measurements were made in HEK 293 or CHO cell lines overexpressing channel subunits and in HUVECs, which natively express Piezo1. Isometric tension recordings were made from rings of mouse thoracic aorta.
Key results: Modification of the pyrazine ring of Yoda1 yielded an analogue, which lacked agonist activity but reversibly antagonized Yoda1. The analogue is referred to as Dooku1. Dooku1 inhibited 2 μM Yoda1-induced Ca2+ -entry with IC50 s of 1.3 μM (HEK 293 cells) and 1.5 μM (HUVECs) yet failed to inhibit constitutive Piezo1 channel activity. It had no effect on endogenous ATP-evoked Ca2+ elevation or store-operated Ca2+ entry in HEK 293 cells or Ca2+ entry through TRPV4 or TRPC4 channels overexpressed in CHO and HEK 293 cells. Yoda1 caused dose-dependent relaxation of aortic rings, which was mediated by an endothelium- and NO-dependent mechanism and which was antagonized by Dooku1 and analogues of Dooku1.
Conclusion and implications: Chemical antagonism of Yoda1-evoked Piezo1 channel activity is possible, and the existence of a specific chemical interaction site is suggested with distinct binding and efficacy domains.
© 2018 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures




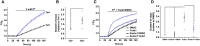



References
-
- Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L et al (2013). Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121: 3925–3935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

