Stopping nucleos(t)ide analogue treatment in Caucasian hepatitis B patients after HBeAg seroconversion is associated with high relapse rates and fatal outcomes
- PMID: 29498078
- PMCID: PMC5900846
- DOI: 10.1111/apt.14560
Stopping nucleos(t)ide analogue treatment in Caucasian hepatitis B patients after HBeAg seroconversion is associated with high relapse rates and fatal outcomes
Abstract
Background: Stopping nucleos(t)ide analogues (NA) after hepatitis B e antigen (HBeAg) seroconversion is associated with high relapse rates in Asian patients, but data in Caucasian cohorts are scarce. Clinical course, outcomes and immunological aspects of chronic hepatitis B infections differ substantially between distinct ethnicities.
Aim: The aim of this study was to determine relapse rates, factors predicting relapse and clinical outcomes after nucleos(t)ide analogue cessation in a large, predominantly Caucasian cohort of chronic hepatitis B patients with nucleos(t)ide analogue-induced HBeAg seroconversion.
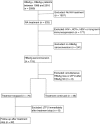
Methods: This is a nationwide observational cohort study including HBeAg positive, mono-infected chronic hepatitis B patients with nucleos(t)ide analogue-induced HBeAg seroconversion from 18 centres in Belgium.
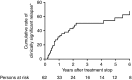
Results: A total of 98 patients with nucleo(s)tide analogue-induced HBeAg seroconversion were included in the study. Of the 62 patients who stopped treatment after a median consolidation treatment of 8 months, 30 relapsed. Higher gamma-glutamyl transferase levels at both treatment initiation (HR 1.004; P = 0.001 per unit increment) and HBeAg seroconversion (HR 1.006; P = 0.013 per unit increment) were associated with an increased risk of clinically significant relapse in a multivariate Cox regression model. Treatment cessation led to liver-related death in 2 patients, of whom one showed a severe flare. Of the patients who continued treatment after HBeAg seroconversion, none relapsed or developed severe hepatic outcomes.
Conclusion: Treatment withdrawal in Caucasian chronic hepatitis B patients after nucleos(t)ide analogue-induced HBeAg seroconversion results in viral relapses in more than half of patients with potential fatal outcomes. These real-world data further lend support to preferentially continue NA treatment after HBeAg seroconversion until HBsAg loss.
© 2018 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
Comment in
-
Editorial: HBeAg seroconversion is not sufficient to allow cessation of nucleos(t)ide analogue treatment in Caucasian hepatitis B patients.Aliment Pharmacol Ther. 2018 Jun;47(11):1551-1552. doi: 10.1111/apt.14649. Aliment Pharmacol Ther. 2018. PMID: 29878428 No abstract available.
References
-
- Chen GF, Wang C, Lau G. Treatment of chronic hepatitis B infection‐2017. Liver Int. 2017;37(Suppl. 1):59‐66. - PubMed
-
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486‐1500. - PubMed
-
- Vanwolleghem T, Hou J, van Oord G, et al. Re‐evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatology. 2015;62:87‐100. - PubMed
-
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370‐398 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
