Quantitative blood flow measurement in rat brain with multiphase arterial spin labelling magnetic resonance imaging
- PMID: 29498562
- PMCID: PMC6681434
- DOI: 10.1177/0271678X18756218
Quantitative blood flow measurement in rat brain with multiphase arterial spin labelling magnetic resonance imaging
Abstract
Cerebral blood flow is an important parameter in many diseases and functional studies that can be accurately measured in humans using arterial spin labelling (ASL) MRI. However, although rat models are frequently used for preclinical studies of both human disease and brain function, rat CBF measurements show poor consistency between studies. This lack of reproducibility is due, partly, to the smaller size and differing head geometry of rats compared to humans, as well as the differing analysis methodologies employed and higher field strengths used for preclinical MRI. To address these issues, we have implemented, optimised and validated a multiphase pseudo-continuous ASL technique, which overcomes many of the limitations of rat CBF measurement. Three rat strains (Wistar, Sprague Dawley and Berlin Druckrey IX) were used, and CBF values validated against gold-standard autoradiography measurements. Label positioning was found to be optimal at 45°, while post-label delay was optimised to 0.55 s. Whole brain CBF measures were 109 ± 22, 111 ± 18 and 100 ± 15 mL/100 g/min by multiphase pCASL, and 108 ± 12, 116 ± 14 and 122 ± 16 mL/100 g/min by autoradiography in Wistar, SD and BDIX cohorts, respectively. Tumour model analysis shows that the developed methods also apply in disease states. Thus, optimised multiphase pCASL provides robust, reproducible and non-invasive measurement of CBF in rats.
Keywords: Arterial spin labelling; autoradiography; cerebral blood flow; multiphase; rats.
Figures
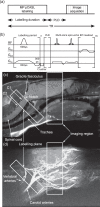



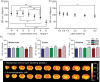

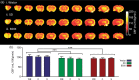
References
-
- Miyaji Y, Yokoyama M, Kawabata Y, et al. Arterial spin-labeling magnetic resonance imaging for diagnosis of late seizure after stroke. J Neurol Sci 2014; 339: 87–90. - PubMed
-
- Zhang S, Yao Y, Zhang S, et al. Comparative study of DSC-PWI and 3D-ASL in ischemic stroke patients. J Huazhong Univ Sci Technolog Med Sci 2015; 35: 923–927. - PubMed
-
- Silva AC, Kim SG, Garwood M. Imaging blood flow in brain tumors using arterial spin labeling. Magn Reson Med 2000; 44: 169–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

