Isolation and Purification of Two Isoflavones from Hericium erinaceum Mycelium by High-Speed Counter-Current Chromatography
- PMID: 29498678
- PMCID: PMC6017085
- DOI: 10.3390/molecules23030560
Isolation and Purification of Two Isoflavones from Hericium erinaceum Mycelium by High-Speed Counter-Current Chromatography
Abstract
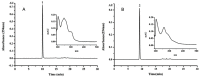
High-speed counter-current chromatography (HSCCC) was used to separate and purify two isoflavones for the first time from Hericium erinaceum (H. erinaceum) mycelium using a two-phase solvent system composed of chloroform-dichloromethane-methanol-water (4:2:3:2, v/v/v/v). These two isoflavones were identified as genistein (4',5,7-trihydroxyisoflavone, C15H10O₅) and daidzein (4',7-dihydroxyisoflavone, C15H10O₄), using infrared spectroscopy (IR), electro-spary ionisation mass (ESI-MS), ¹H-nuclear magnetic resonance (NMR) and 13C-NMR spectra. About 23 mg genistein with 95.7% purity and 18 mg daidzein with 97.3% purity were isolated from 150 mg ethanolic extract of H. erinaceum mycelium. The results demonstrated that HSCCC was a feasible method to separate and purify genistein and daidzein from H. erinaceum mycelium.
Keywords: Hericium erinaceuns mycelium; daidzein; genistein; high-speed counter-current chromatography (HSCCC).
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Chang C.H., Chen Y., Yew X.X., Chen H.X., Kim J.X., Chang C.C., Peng C.C., Peng R.Y. Improvement of Erinacine A Productivity in Hericium erinaceus Mycelia and Its Neuroprotective Bioactivity against the Glutamate-Insulted Apoptosis. LWT Food Sci. Technol. 2016;19:616–626. doi: 10.1016/j.lwt.2015.08.014. - DOI
-
- Mizuno T. Yamabushitake, Hericium erinaceum: Bioactive substances and medicinal utilization. Food Rev. Int. 1995;11:173–178. doi: 10.1080/87559129509541027. - DOI
-
- Okamoto K., Sakai T., Shimada A., Shirai R., Sakamoto H., Yoshida S., Ojima F., Ishiguro Y., Kawagishi H. Antimicrobial chlorinated orcinol derivatives from mycelia of Hericium erinaceum. Phytochemistry. 1993;34:1445–1446. doi: 10.1016/0031-9422(91)80050-B. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

