Complexes of gold and imidazole formed in helium nanodroplets
- PMID: 29498720
- PMCID: PMC5885785
- DOI: 10.1039/c8cp00486b
Complexes of gold and imidazole formed in helium nanodroplets
Abstract
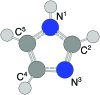
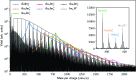
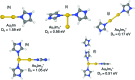
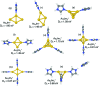
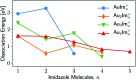
We have studied complexes of gold atoms and imidazole (C3N2H4, abbreviated Im) produced in helium nanodroplets. Following the ionization of the doped droplets we detect a broad range of different AumImn+ complexes, however we find that for specific values of m certain n are "magic" and thus particularly abundant. Our density functional theory calculations indicate that these abundant clusters sizes are partially the result of particularly stable complexes, e.g. AuIm2+, and partially due to a transition in fragmentation patterns from the loss of neutral imidazole molecules for large systems to the loss of neutral gold atoms for smaller systems.
Figures




References
-
- Schwarz H. Angew. Chem., Int. Ed. 2003;42:4442–4454. - PubMed
-
- Brown C. M., Ginter M. L. J. Opt. Soc. Am. 1978;68:243–246.
-
- Andersen T., Haugen H. K., Hotop H. J. Phys. Chem. Ref. Data. 1999;28:1511–1533.
-
- Zeller E., Beruda H., Kolb A., Bissinger P., Riede J., Schmidbaur H. Nature. 1991;352:141–143.
-
- Schmidbaur H., Steigelmann O. Z. Naturforsch. B. 1992;47:1721.
LinkOut - more resources
Full Text Sources
Other Literature Sources

