Optogenetically controlled protein kinases for regulation of cellular signaling
- PMID: 29498733
- PMCID: PMC5882534
- DOI: 10.1039/c7cs00404d
Optogenetically controlled protein kinases for regulation of cellular signaling
Abstract
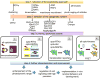
Protein kinases are involved in the regulation of many cellular processes including cell differentiation, survival, migration, axon guidance and neuronal plasticity. A growing set of optogenetic tools, termed opto-kinases, allows activation and inhibition of different protein kinases with light. The optogenetic regulation enables fast, reversible and non-invasive manipulation of protein kinase activities, complementing traditional methods, such as treatment with growth factors, protein kinase inhibitors or chemical dimerizers. In this review, we summarize the properties of the existing optogenetic tools for controlling tyrosine kinases and serine-threonine kinases. We discuss how the opto-kinases can be applied for studies of spatial and temporal aspects of protein kinase signaling in cells and organisms. We compare approaches for chemical and optogenetic regulation of protein kinase activity and present guidelines for selection of opto-kinases and equipment to control them with light. We also describe strategies to engineer novel opto-kinases on the basis of various photoreceptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

