Differential effects of Vav-promoter-driven overexpression of BCLX and BFL1 on lymphocyte survival and B cell lymphomagenesis
- PMID: 29498802
- PMCID: PMC5947286
- DOI: 10.1111/febs.14426
Differential effects of Vav-promoter-driven overexpression of BCLX and BFL1 on lymphocyte survival and B cell lymphomagenesis
Abstract
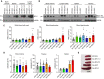
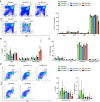
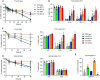
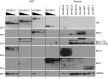
Overexpression of BCLX and BFL1/A1 has been reported in various human malignancies and is associated with poor prognosis and drug resistance, identifying these prosurvival BCL2 family members as putative drug targets. We have generated transgenic mice that express human BFL1 or human BCLX protein throughout the haematopoietic system under the control of the Vav gene promoter. Haematopoiesis is normal in both the Vav-BFL1 and Vav-BCLX transgenic (TG) mice and susceptibility to spontaneous haematopoietic malignancies is not increased. Lymphoid cells from Vav-BCLX TG mice exhibit increased resistance to apoptosis in vitro while most blood cell types form Vav-BFL1 TG mice were poorly protected. Both transgenes significantly accelerated lymphomagenesis in Eμ-MYC TG mice and, surprisingly, the Vav-BFL1 transgene was the more potent. Unexpectedly, expression of transgenic BFL1 RNA and protein is significantly elevated in B lymphoid cells of Vav-BFL1/Eμ-MYC double-transgenic compared to Vav-BFL1 mice, even during the preleukaemic phase, providing a rationale for the potent synergy. In contrast, Vav-BCLX expression was not notably different. These mouse models of BFL1 and BCLX overexpression in lymphomas should be useful tools for the testing the efficacy of novel human BFL1- and BCLX-specific inhibitors.
Keywords: BCLX; BFL1/A1; MYC; Vav-promoter; apoptosis; lymphomagenesis.
© 2018 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures



Similar articles
-
MYC selects against reduced BCL2A1/A1 protein expression during B cell lymphomagenesis.Oncogene. 2017 Apr;36(15):2066-2073. doi: 10.1038/onc.2016.362. Epub 2016 Oct 3. Oncogene. 2017. PMID: 27694901 Free PMC article.
-
BFL1 modulates apoptosis at the membrane level through a bifunctional and multimodal mechanism showing key differences with BCLXL.Cell Death Differ. 2019 Oct;26(10):1880-1894. doi: 10.1038/s41418-018-0258-5. Epub 2018 Dec 18. Cell Death Differ. 2019. PMID: 30560933 Free PMC article.
-
IBTK contributes to B-cell lymphomagenesis in Eμ-myc transgenic mice conferring resistance to apoptosis.Cell Death Dis. 2019 Apr 11;10(4):320. doi: 10.1038/s41419-019-1557-6. Cell Death Dis. 2019. PMID: 30975981 Free PMC article.
-
Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: implications for targeted therapy.FEBS J. 2015 Mar;282(5):834-849. doi: 10.1111/febs.13188. Epub 2015 Jan 23. FEBS J. 2015. PMID: 25559680 Free PMC article. Review.
-
Transgenic models of lymphoid neoplasia and development of a pan-hematopoietic vector.Oncogene. 1999 Sep 20;18(38):5268-77. doi: 10.1038/sj.onc.1202997. Oncogene. 1999. PMID: 10498879 Review.
Cited by
-
Mouse Models of c-myc Deregulation Driven by IgH Locus Enhancers as Models of B-Cell Lymphomagenesis.Front Immunol. 2020 Jul 23;11:1564. doi: 10.3389/fimmu.2020.01564. eCollection 2020. Front Immunol. 2020. PMID: 32793219 Free PMC article. Review.
-
Inhibition of bromodomain and extra-terminal proteins targets constitutively active NFκB and STAT signaling in lymphoma and influences the expression of the antiapoptotic proteins BCL2A1 and c-MYC.Cell Commun Signal. 2024 Aug 27;22(1):415. doi: 10.1186/s12964-024-01782-9. Cell Commun Signal. 2024. PMID: 39192247 Free PMC article.
-
Last but not least: BFL-1 as an emerging target for anti-cancer therapies.Biochem Soc Trans. 2022 Aug 31;50(4):1119-1128. doi: 10.1042/BST20220153. Biochem Soc Trans. 2022. PMID: 35900226 Free PMC article. Review.
References
-
- Lin EY, Orlofsky A, Berger MS & Prystowsky MB (1993) Characterization of A1, a novel hemopoietic‐specific early‐response gene with sequence similarity to bcl‐2. J Immunol 151, 1979–1988. - PubMed
-
- Orlofsky A, Somogyi RD, Weiss LM & Prystowsky MB (1999) The murine antiapoptotic protein A1 is induced in inflammatory macrophages and constitutively expressed in neutrophils. J Immunol 163, 412–419. - PubMed
-
- Verschelde C, Walzer T, Galia P, Biemont MC, Quemeneur L, Revillard JP, Marvel J & Bonnefoy‐Berard N (2003) A1/Bfl‐1 expression is restricted to TCR engagement in T lymphocytes. Cell Death Differ 10, 1059–1067. - PubMed
-
- Hatakeyama S, Hamasaki A, Negishi I, Loh DY, Sendo F & Nakayama K (1998) Multiple gene duplication and expression of mouse bcl‐2‐related genes, A1. Int Immunol 10, 631–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

