Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development
- PMID: 29499151
- PMCID: PMC5837068
- DOI: 10.1016/j.stem.2018.02.011
Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development
Abstract
Organs-on-a-chip (OOCs) are miniature tissues and organs grown in vitro that enable modeling of human physiology and disease. The technology has emerged from converging advances in tissue engineering, semiconductor fabrication, and human cell sourcing. Encompassing innovations in human stem cell technology, OOCs offer a promising approach to emulate human patho/physiology in vitro, and address limitations of current cell and animal models. Here, we review the design considerations for single and multi-organ OOCs, discuss remaining challenges, and highlight the potential impact of OOCs as a fast-track opportunity for tissue engineering to advance drug development and precision medicine.
Keywords: disease modeling; drug development; human physiology; human stem cells; microfluidics; precision medicine; preclinical studies; tissue engineering.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors are co-founders of Tara Biosystems, a Columbia University startup company commercializing organs on a chip with human heart muscle.
Figures


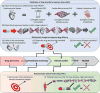
References
-
- Ahadian S, Civitarese R, Bannerman D, Mohammadi MH, Lu R, Wang E, Davenport-Huyer L, Lai B, Zhang B, Zhao Y, Mandla S, Korolj A, Radisic M. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv Healthc Mater 2017 - PubMed
-
- Ahmad S, Wolfe S. Cisapride and torsades de pointes. The Lancet. 345:508.
-
- Atac B, Wagner I, Horland R, Lauster R, Marx U, Tonevitsky AG, Azar RP, Lindner G. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip. 2013;13:3555–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

