The Transcriptionally Permissive Chromatin State of Embryonic Stem Cells Is Acutely Tuned to Translational Output
- PMID: 29499153
- PMCID: PMC5836508
- DOI: 10.1016/j.stem.2018.02.004
The Transcriptionally Permissive Chromatin State of Embryonic Stem Cells Is Acutely Tuned to Translational Output
Abstract
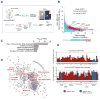
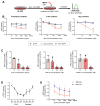
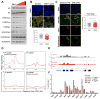
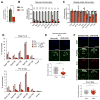
A permissive chromatin environment coupled to hypertranscription drives the rapid proliferation of embryonic stem cells (ESCs) and peri-implantation embryos. We carried out a genome-wide screen to systematically dissect the regulation of the euchromatic state of ESCs. The results revealed that cellular growth pathways, most prominently translation, perpetuate the euchromatic state and hypertranscription of ESCs. Acute inhibition of translation rapidly depletes euchromatic marks in mouse ESCs and blastocysts, concurrent with delocalization of RNA polymerase II and reduction in nascent transcription. Translation inhibition promotes rewiring of chromatin accessibility, which decreases at a subset of active developmental enhancers and increases at histone genes and transposable elements. Proteome-scale analyses revealed that several euchromatin regulators are unstable proteins and continuously depend on a high translational output. We propose that this mechanistic interdependence of euchromatin, transcription, and translation sets the pace of proliferation at peri-implantation and may be employed by other stem/progenitor cells.
Keywords: Chd1; blastocyst; embryonic stem cells; euchromatin; hypertranscription; mTOR; permissive chromatin; ribosome; translation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Living Up to the Hype: Protein Synthesis Promotes Hypertranscription in Embryonic Stem Cells.Cell Stem Cell. 2018 Mar 1;22(3):281-282. doi: 10.1016/j.stem.2018.02.008. Cell Stem Cell. 2018. PMID: 29499142
-
Stem cells: Translating hypertranscription in embryonic stem cells.Nat Rev Mol Cell Biol. 2018 Mar 20;19(4):209. doi: 10.1038/nrm.2018.19. Nat Rev Mol Cell Biol. 2018. PMID: 29556087 No abstract available.
-
A translational rheostat integrates euchromatin regulation and growth of pluripotent embryonic cells.Stem Cell Investig. 2019 May 21;6:11. doi: 10.21037/sci.2019.05.01. eCollection 2019. Stem Cell Investig. 2019. PMID: 31231668 Free PMC article. No abstract available.
-
Tuning the chromatin landscape of embryonic stem cells.Stem Cell Investig. 2019 Jul 2;6:16. doi: 10.21037/sci.2019.06.02. eCollection 2019. Stem Cell Investig. 2019. PMID: 31463309 Free PMC article. No abstract available.
References
-
- Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Čech M, Chilton J, Clements D, Coraor N, Eberhard C, Grüning B, Guerler A, Hillman-Jackson J, Kuster Von G, Rasche E, Soranzo N, Turaga N, Taylor J, Nekrutenko A, Goecks J. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44:W3–W10. doi: 10.1093/nar/gkw343. - DOI - PMC - PubMed
-
- Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, Wang J, Rendl M, Bernstein E, Schaniel C, Lemischka IR. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

