CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts
- PMID: 29499171
- PMCID: PMC6119541
- DOI: 10.1016/j.jalz.2018.01.010
CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts
Abstract
Introduction: We studied whether fully automated Elecsys cerebrospinal fluid (CSF) immunoassay results were concordant with positron emission tomography (PET) and predicted clinical progression, even with cutoffs established in an independent cohort.
Methods: Cutoffs for Elecsys amyloid-β1-42 (Aβ), total tau/Aβ(1-42), and phosphorylated tau/Aβ(1-42) were defined against [18F]flutemetamol PET in Swedish BioFINDER (n = 277) and validated against [18F]florbetapir PET in Alzheimer's Disease Neuroimaging Initiative (n = 646). Clinical progression in patients with mild cognitive impairment (n = 619) was studied.
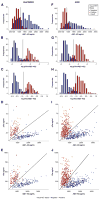
Results: CSF total tau/Aβ(1-42) and phosphorylated tau/Aβ(1-42) ratios were highly concordant with PET classification in BioFINDER (overall percent agreement: 90%; area under the curve: 94%). The CSF biomarker statuses established by predefined cutoffs were highly concordant with PET classification in Alzheimer's Disease Neuroimaging Initiative (overall percent agreement: 89%-90%; area under the curves: 96%) and predicted greater 2-year clinical decline in patients with mild cognitive impairment. Strikingly, tau/Aβ ratios were as accurate as semiquantitative PET image assessment in predicting visual read-based outcomes.
Discussion: Elecsys CSF biomarker assays may provide reliable alternatives to PET in Alzheimer's disease diagnosis.
Keywords: Amyloid PET concordance; Amyloid-β (1–42); Biomarker validation; CSF biomarkers; Clinical progression; Cutoffs; Phosphorylated tau (pTau); Total tau (tTau).
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. - PubMed
-
- Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388:505–17. - PubMed
-
- American Academy of Neurology. [Accessed March 5, 2018];AAN Guideline Summary for clinicians: detection, diagnosis and management of dementia. 2004. 2014 Jul; Available at: http://tools.aan.com/professionals/practice/pdfs/dementia_guideline.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

