The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice
- PMID: 29499566
- PMCID: PMC5952914
- DOI: 10.1016/j.redox.2018.02.022
The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice
Erratum in
-
Corrigendum to "The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice" [Redox Biol. 16 (2018) 129-138].Redox Biol. 2024 Jun;72:103163. doi: 10.1016/j.redox.2024.103163. Epub 2024 Apr 23. Redox Biol. 2024. PMID: 38653649 Free PMC article. No abstract available.
-
Corrigendum to "The Immunoproteasome Subunit LMP10 Mediates Angiotensin II-induced Retinopathy in Mice" [Redox Biol. 16 (2018) 129-138].Redox Biol. 2024 Jul;73:103181. doi: 10.1016/j.redox.2024.103181. Epub 2024 May 10. Redox Biol. 2024. PMID: 38729784 Free PMC article. No abstract available.
Abstract
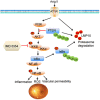
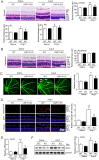
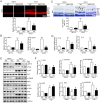
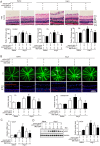
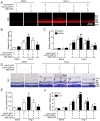
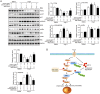
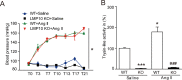
Inflammation has been implicated in a variety of retinal diseases. The immunoproteasome plays a critical role in controlling inflammatory responses, but whether activation of immunoproteasome contributes to angiotensin II (Ang II)-induced retinopathy remains unclear. Hypertensive retinopathy (HR) was induced by infusion of Ang II (3000 ng/kg/min) in wild-type (WT) and immunoproteasome subunit LMP10 knockout (KO) mice for 3 weeks. Changes in retinal morphology, vascular permeability, superoxide production and inflammation were examined by pathological staining. Our results showed that immunoproteasome subunit LMP10 expression and its trypsin-like activity were significantly upregulated in the retinas and serum of Ang II-infused mice and in the serum from patients with hypertensive retinopathy. Moreover, Ang II-infused WT mice showed an increase in the central retinal thickness, vascular permeability, reactive oxygen species (ROS) production and inflammation compared with saline controls, and these effects were significantly attenuated in LMP10 KO mice, but were aggravated in mice intravitreally injected with rAAV2-LMP10. Interestingly, administration of IKKβ specific inhibitor IMD-0354 remarkably blocked an Ang II-induced increase in vascular permeability, oxidative stress and inflammation during retinopathy. Mechanistically, Ang II-induced upregulation of LMP10 promoted PTEN degradation and activation of AKT/IKK signaling, which induced IkBα phosphorylation and subsequent degradation ultimately leading to activation of NF-kB target genes in retinopathy. Therefore, this study provided novel evidence demonstrating that LMP10 is a positive regulator of NF-kB signaling, which contributes to Ang II-induced retinopathy. Strategies for inhibiting LMP10 or IKKβ activity in the eye could serve as a novel therapeutic target for treating hypertensive retinopathy.
Keywords: Angiotensin II; Immunoproteasome LMP10; Inflammation; Oxidative stress; Retinopathy; Vascular permeability.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Katsi V., Marketou M., Vlachopoulos C. Impact of arterial hypertension on the eye. Curr. Hypertens. Rep. 2012;14:581–590. - PubMed
-
- Wong T.Y., McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br. Med. Bull. 2005;73–74:57–70. - PubMed
-
- Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38:752–765. - PubMed
-
- Louie J.L., Kapphahn R.J., Ferrington D.A. Proteasome function and protein oxidation in the aged retina. Exp. Eye Res. 2002;75:271–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

