The Strengthening Exercises in Shoulder Impingement trial (The SExSI-trial) investigating the effectiveness of a simple add-on shoulder strengthening exercise programme in patients with long-lasting subacromial impingement syndrome: Study protocol for a pragmatic, assessor blinded, parallel-group, randomised, controlled trial
- PMID: 29499710
- PMCID: PMC5833202
- DOI: 10.1186/s13063-018-2509-7
The Strengthening Exercises in Shoulder Impingement trial (The SExSI-trial) investigating the effectiveness of a simple add-on shoulder strengthening exercise programme in patients with long-lasting subacromial impingement syndrome: Study protocol for a pragmatic, assessor blinded, parallel-group, randomised, controlled trial
Abstract
Background: Subacromial impingement syndrome (SIS) is a painful, and often long lasting, shoulder condition affecting patient function and quality of life. In a recent study, we observed major strength impairments in shoulder external rotation and abduction (~30%) in a population of patients with pronounced and long-lasting SIS. However, the current rehabilitation of such strength impairments may be inadequate, with novel rehabilitation programmes including exercise therapy only improving external rotation strength by 4-13%. As these previous studies are the basis of current practice, this suggests that the strengthening component could be inadequate in the rehabilitation of these patients, and it seems likely that more emphasis should be placed on intensifying this part of the rehabilitation. The purpose of this study is to investigate the effectiveness of a programme consisting of progressive home-based resistance training using an elastic band, aimed at improving shoulder external rotation and abduction strength, added to usual care and initiated shortly after diagnosis has been established.
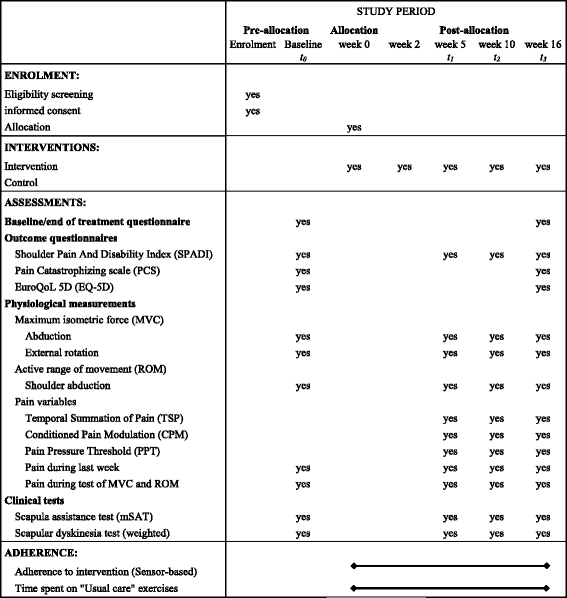
Methods: A pragmatic randomised controlled superiority trial will be conducted, including 200 patients with pronounced and long-lasting SIS, diagnosed using predefined criteria. Participants will be randomised to receive either an add-on intervention of progressive home-based resistance training using an elastic band in addition to usual care or usual care alone in a 1:1 allocation ratio. The randomisation sequence is computer generated, with permuted blocks of random sizes. The primary outcome will be change in Shoulder Pain And Disability Index (SPADI) score from baseline to 16 weeks follow-up. Outcome assessors are blinded to group allocation. Intervention receivers will be kept blind to treatment allocation through minimal information about the content of the add-on intervention and control condition until group allocation is final. Analyses are performed by blinded data analysts.
Discussion: If effective, the simple shoulder strengthening exercise programme investigated in this trial could easily be added to usual care. The usefulness of the trial is further supported by the magnitude of the problem, the information gained from the study and the pragmatism, patient centeredness and transparency of the trial.
Trial registration: The trial is pre-registered at ClinicalTrials.gov with the ID NCT02747251 on April 19, 2016.
Keywords: Adherence; Exercise; Impingement; Pragmatic; Progressive; RCT; Rotator cuff; Sensitisation; Shoulder; Strength.
Conflict of interest statement
Ethics approval and consent to participate
The trial protocol, the informed consent forms and other requested documents have been reviewed and approved by the Capitol Regional Ethics Committee in Denmark (H-16016763) with respect to the scientific content and the compliance to the applicable health science regulations. Safety reports, containing a list of all serious side effects and serious events, will be sent to the ethics committee. Within 90 days after completion of the study, the sponsor and the primary investigator will inform the ethics committee that the study is closed.
At the end of the medical examination, the treating orthopaedic specialist or the cooperating nurse will introduce the trial to patients fulfilling the eligibility criteria. Patients will be informed about the request for participation in the trial, and will be provided with participant information and a brochure on test subject’s rights. As soon as possible thereafter, patients will be contacted by one of the investigators. A final eligibility screening will be conducted and eligible patients will be offered a meeting to provide oral information about the trial, their rights as test subjects and the possibility to ask clarifying questions. Patients are offered deliberation time before being asked to sign written consent forms regarding willingness to participate.
In the oral information and in the participant information sheets, patients will be informed that a 1 year follow-up is planned for all participants, and that this is covered by the written consent forms. Data for the 1 year follow-up will, in part, be collected through text messages, using the SMS-track© (data hosted at a BFIH certified data centre that meets the ISO 27002 standards). Through answers to auto-generated standardized SMS questions, patients will be asked to report sick leave due to their shoulder disorder (weekly). Questions regarding surgery, if any, for SIS (yearly), the EQ-5D-3 L questionnaire (yearly), and all questions from the SPADI questionnaire (after 6 months and 1 year) will be administered using the REDCap® system.
All study-related information on participants will be stored securely on the study site. All completed paper forms will be stored in locked file cabinets before and after data is entered to an electronic database. All electronic participants’ information will be stored on a secured study-specific drive at the study site. Access to the study-specific drive will be limited to users currently working on the project and therefore need access to the secured drive in order to store and/or analyse data. Access will be limited by individual person-specific login and passwords. A list of persons with access to the study-specific drive will be kept up-to-date by the primary investigator. Appointment schedules and any other listings that link participant to other identifying information will be stored separately in the hospital’s secured intranet system or in separate locked file cabinets in an area with limited access. Study information will not be released outside of the study without the permission of the relevant participant. The study will be reported to and approved by the Danish Data Protection Agency before start of inclusion, and will adhere to the “Act on Processing Personal Data”.
In general, no ancillary or post-trial care is provided to participants in the trial. However, the details of the intervention will be made public shortly after the trial is over, and the intervention could therefore be replicated by physiotherapists in ordinary settings.
Consent for publication
Written informed consent for publication of images in the intervention leaflet was obtained from the person appearing on the pictures. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial).Am J Sports Med. 2021 Sep;49(11):3040-3049. doi: 10.1177/03635465211016008. Epub 2021 May 28. Am J Sports Med. 2021. PMID: 34048281 Free PMC article. Clinical Trial.
-
Glenohumeral and scapulothoracic strength impairments exists in patients with subacromial impingement, but these are not reflected in the shoulder pain and disability index.BMC Musculoskelet Disord. 2017 Jul 17;18(1):302. doi: 10.1186/s12891-017-1667-1. BMC Musculoskelet Disord. 2017. PMID: 28716019 Free PMC article.
-
Effectiveness of individualized physiotherapy on pain and functioning compared to a standard exercise protocol in patients presenting with clinical signs of subacromial impingement syndrome. A randomized controlled trial.BMC Musculoskelet Disord. 2010 Jun 9;11:114. doi: 10.1186/1471-2474-11-114. BMC Musculoskelet Disord. 2010. PMID: 20534140 Free PMC article. Clinical Trial.
-
Progressive resistance training in patients with shoulder impingement syndrome: literature review.Reumatismo. 2009 Apr-Jun;61(2):84-9. doi: 10.4081/reumatismo.2009.84. Reumatismo. 2009. PMID: 19633794 Review.
-
Effect of supervised physiotherapy versus home exercise program in patients with subacromial impingement syndrome: A systematic review and meta-analysis.Phys Ther Sport. 2020 Jan;41:34-42. doi: 10.1016/j.ptsp.2019.11.003. Epub 2019 Nov 6. Phys Ther Sport. 2020. PMID: 31726386
Cited by
-
Evaluation of the Efficacy and Safety of Silver Nanoparticles in the Treatment of Non-Neurological and Neurological Distemper in Dogs: A Randomized Clinical Trial.Viruses. 2022 Oct 24;14(11):2329. doi: 10.3390/v14112329. Viruses. 2022. PMID: 36366427 Free PMC article.
-
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial).Am J Sports Med. 2021 Sep;49(11):3040-3049. doi: 10.1177/03635465211016008. Epub 2021 May 28. Am J Sports Med. 2021. PMID: 34048281 Free PMC article. Clinical Trial.
-
Level of pain catastrophising determines if patients with long-standing subacromial impingement benefit from more resistance exercise: predefined secondary analyses from a pragmatic randomised controlled trial (the SExSI Trial).Br J Sports Med. 2023 Jul;57(13):842-848. doi: 10.1136/bjsports-2022-106383. Epub 2023 Mar 10. Br J Sports Med. 2023. PMID: 36898767 Free PMC article. Clinical Trial.
-
Hip strengthening exercise dosage is not associated with clinical improvements after total hip arthroplasty - a prospective cohort study (the PHETHAS-1 study).BMC Musculoskelet Disord. 2024 Nov 19;25(1):928. doi: 10.1186/s12891-024-08057-x. BMC Musculoskelet Disord. 2024. PMID: 39563311 Free PMC article.
-
Shoulder Rehabilitation Exercises With Kinematic Biofeedback After Arthroscopic Rotator Cuff Repair: Protocol for a New Integrated Rehabilitation Program.JMIR Res Protoc. 2023 Mar 22;12:e35757. doi: 10.2196/35757. JMIR Res Protoc. 2023. PMID: 36947146 Free PMC article.
References
-
- Sundhedsstyrelsen . Impingementsyndrom/rotator cuff-syndrom og traumatisk rotator cuff-ruptur Del 2: Faglige visitationsretningslinjer. København: Sundhedsstyrelsen; 2011.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous