White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes
- PMID: 29499767
- PMCID: PMC5834839
- DOI: 10.1186/s40478-018-0515-3
White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes
Abstract
Alzheimer's disease (AD) is conceptualized as a progressive consequence of two hallmark pathological changes in grey matter: extracellular amyloid plaques and neurofibrillary tangles. However, over the past several years, neuroimaging studies have implicated micro- and macrostructural abnormalities in white matter in the risk and progression of AD, suggesting that in addition to the neuronal pathology characteristic of the disease, white matter degeneration and demyelination may be also important pathophysiological features. Here we review the evidence for white matter abnormalities in AD with a focus on myelin and oligodendrocytes, the only source of myelination in the central nervous system, and discuss the relationship between white matter changes and the hallmarks of Alzheimer's disease. We review several mechanisms such as ischemia, oxidative stress, excitotoxicity, iron overload, Aβ toxicity and tauopathy, which could affect oligodendrocytes. We conclude that white matter abnormalities, and in particular myelin and oligodendrocytes, could be mechanistically important in AD pathology and could be potential treatment targets.
Keywords: Alzheimer’s disease; Myelin, Oligodendrocyte; Neurodegeneration; Oxidative stress; White matter.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
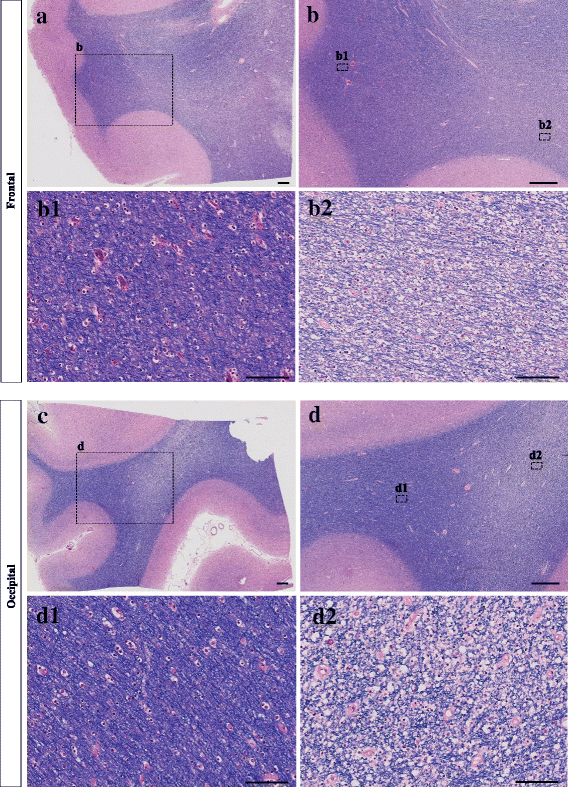
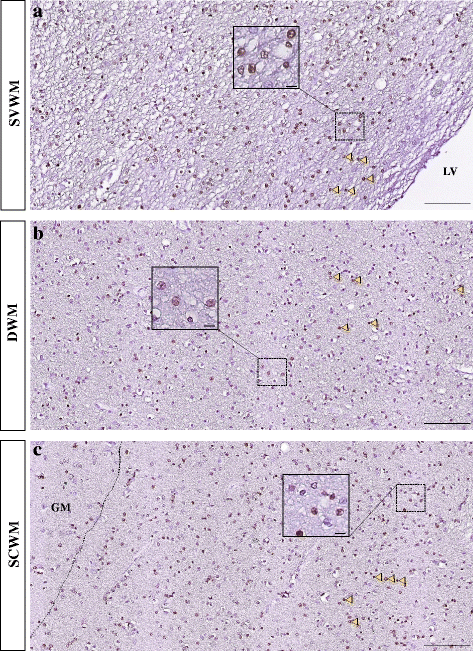
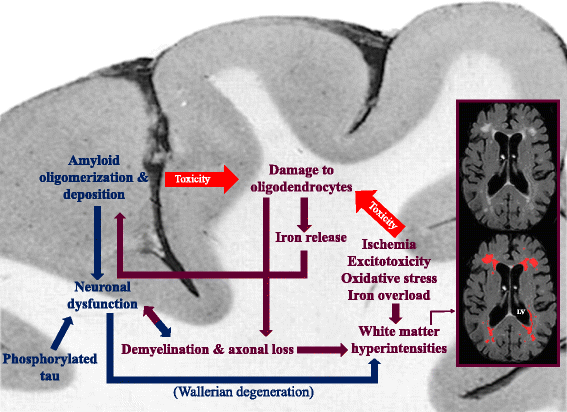
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

