Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA)
- PMID: 29500065
- PMCID: PMC6090941
- DOI: 10.1016/j.eururo.2018.02.013
Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA)
Abstract
Background: Noninvasive biomarkers are needed to guide metastatic castration-resistant prostate cancer (mCRPC) treatment.
Objective: To clinically qualify baseline and on-treatment cell-free DNA (cfDNA) concentrations as biomarkers of patient outcome following taxane chemotherapy.
Design, setting, and participants: Blood for cfDNA analyses was prospectively collected from 571 mCRPC patients participating in two phase III clinical trials, FIRSTANA (NCT01308567) and PROSELICA (NCT01308580). Patients received docetaxel (75mg/m2) or cabazitaxel (20 or 25mg/m2) as first-line chemotherapy (FIRSTANA), and cabazitaxel (20 or 25mg/m2) as second-line chemotherapy (PROSELICA).
Outcome measurements and statistical analysis: Associations between cfDNA concentration and prostate-specific antigen (PSA) response were tested using logistic regression models. Survival was estimated using Kaplan-Meier methods for cfDNA concentration grouped by quartile. Cox proportional hazard models, within each study, tested for associations with radiological progression-free survival (rPFS) and overall survival (OS), with multivariable analyses adjusting for baseline prognostic variables. Two-stage individual patient meta-analysis combined results for cfDNA concentrations for both studies.
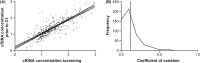
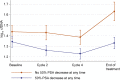
Results and limitations: In 2502 samples, baseline log10 cfDNA concentration correlated with known prognostic factors, shorter rPFS (hazard ratio [HR]=1.54; 95% confidence interval [CI]: 1.15-2.08; p=0.004), and shorter OS on taxane therapy (HR=1.53; 95% CI: 1.18-1.97; p=0.001). In multivariable analyses, baseline cfDNA concentration was an independent prognostic variable for rPFS and OS in both first- and second-line chemotherapy settings. Patients with a PSA response experienced a decline in log10 cfDNA concentrations during the first four cycles of treatment (per cycle -0.03; 95% CI: -0.044 to -0.009; p=0.003). Study limitations included the fact that blood sample collection was not mandated for all patients and the inability to specifically quantitate tumour-derived cfDNA fraction in cfDNA.
Conclusions: We report that changes in cfDNA concentrations correlate with both rPFS and OS in patients receiving first- and second-line taxane therapy, and may serve as independent prognostic biomarkers of response to taxanes.
Patient summary: In the past decade, several new therapies have been introduced for men diagnosed with metastatic prostate cancer. Although metastatic prostate cancer remains incurable, these novel agents have extended patient survival and improved their quality of life in comparison with the last decade. To further optimise treatment allocation and individualise patient care, better tests (biomarkers) are needed to guide the delivery of improved and more precise care. In this report, we assessed cfDNA in over 2500 blood samples from men with prostate cancer who were recruited to two separate international studies and received taxane chemotherapy. We quantified the concentration of cfDNA fragments in blood plasma, which partly originates from tumour. We identified that higher concentrations of circulating cfDNA fragments, prior to starting taxane chemotherapy, can be used to identify patients with aggressive prostate cancer. A decline in cfDNA concentration during the first 3-9 wk after initiation of taxane therapy was seen in patients deriving benefit from taxane chemotherapy. These results identified circulating cfDNA as a new biomarker of aggressive disease in metastatic prostate cancer and imply that the study of cfDNA has clinical utility, supporting further efforts to develop blood-based tests on this circulating tumour-derived DNA.
Keywords: Cabazitaxel; Circulating cell-free DNA; Docetaxel; FIRSTANA; PROSELICA; Taxane chemotherapy; cfDNA.
Copyright © 2018 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Cell-free DNA in Advanced Prostate Cancer: A Biomarker Revolution Under Way?Eur Urol. 2018 Sep;74(3):292-293. doi: 10.1016/j.eururo.2018.03.002. Epub 2018 Mar 19. Eur Urol. 2018. PMID: 29566958 No abstract available.
-
Re: Niven Mehra, David Dolling, Semini Sumanasuriya, et al. Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA). Eur Urol 2018;74:283-91.Eur Urol. 2018 Sep;74(3):e67-e68. doi: 10.1016/j.eururo.2018.05.015. Epub 2018 May 24. Eur Urol. 2018. PMID: 29803584 No abstract available.
-
Reply to Vincenza Conteduca, Giorgia Gurioli, and Ugo De Giorgi's Letter to the Editor re: Niven Mehra, David Dolling, Semini Sumanasuriya, et al. Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA). Eur Urol. In press. https://doi.org/10.1016/j.eururo.2018.02.013.Eur Urol. 2018 Sep;74(3):e69-e70. doi: 10.1016/j.eururo.2018.05.016. Epub 2018 Jun 1. Eur Urol. 2018. PMID: 29866468 No abstract available.
References
-
- Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; 2013; Lyon, France: 2016. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancer base no. 11.
-
- Tannock I.F., de Wit R., Berry W.R. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Petrylak D.P., Tangen C.M., Hussain M.H. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- de Bono J.S., Oudard S., Ozguroglu M. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. - PubMed
-
- Sweeney C., Chen Y., Liu G. Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. Ann Oncol. 2016;27(Suppl 6):720PD.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

