SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond
- PMID: 29500098
- PMCID: PMC5923433
- DOI: 10.1016/j.tibs.2018.01.005
SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond
Abstract
Sterol regulatory element-binding proteins (SREBPs) are a family of membrane-bound transcription factors that activate genes encoding enzymes required for synthesis of cholesterol and unsaturated fatty acids. SREBPs are controlled by multiple mechanisms at the level of mRNA synthesis, proteolytic activation, and transcriptional activity. In this review, we summarize the recent findings that contribute to the current understanding of the regulation of SREBPs and their physiologic roles in maintenance of lipid homeostasis, insulin signaling, innate immunity, and cancer development.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
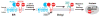



References
-
- Yokoyama C, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75(1):187–197. - PubMed
-
- Wang X, et al. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. - PubMed
-
- Hua X, et al. Hairpin orientation of sterol regulatory element-binding protein-2 in cell membranes as determined by protease protection. Journal of Biological Chemistry. 1995;270(49):29422–29427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

