Spin-controlled atom-ion chemistry
- PMID: 29500464
- PMCID: PMC5834540
- DOI: 10.1038/s41467-018-03373-y
Spin-controlled atom-ion chemistry
Erratum in
-
Publisher Correction: Spin-controlled atom-ion chemistry.Nat Commun. 2018 Apr 23;9(1):1669. doi: 10.1038/s41467-018-04165-0. Nat Commun. 2018. PMID: 29686374 Free PMC article.
Abstract
Quantum control of chemical reactions is an important goal in chemistry and physics. Ultracold chemical reactions are often controlled by preparing the reactants in specific quantum states. Here we demonstrate spin-controlled atom-ion inelastic (spin-exchange) processes and chemical (charge-exchange) reactions in an ultracold Rb-Sr+ mixture. The ion's spin state is controlled by the atomic hyperfine spin state via spin-exchange collisions, which polarize the ion's spin parallel to the atomic spin. We achieve ~ 90% spin polarization due to the absence of strong spin-relaxation channel. Charge-exchange collisions involving electron transfer are only allowed for (RbSr)+ colliding in the singlet manifold. Initializing the atoms in various spin states affects the overlap of the collision wave function with the singlet molecular manifold and therefore also the reaction rate. Our observations agree with theoretical predictions.
Conflict of interest statement
The authors declare no competing interests.
Figures


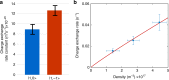
References
-
- Chin C, Grimm R, Julienne P, Tiesinga E. Feshbach resonances in ultracold gases. Rev. Mod. Phys. 2010;82:1225–1286. doi: 10.1103/RevModPhys.82.1225. - DOI
-
- Jones KM, Tiesinga E, Lett PD, Julienne PS. Ultracold photoassociation spectroscopy: long-range molecules and atomic scattering. Rev. Mod. Phys. 2006;78:483–535. doi: 10.1103/RevModPhys.78.483. - DOI
-
- Härter A, Hecker Denschlag J. Cold atom-ion experiments in hybrid traps. Contemp. Phys. 2014;55:33–45. doi: 10.1080/00107514.2013.854618. - DOI
-
- Ratschbacher L, Zipkes C, Sias C, Köhl M. Controlling chemical reactions of a single particle. Nat. Phys. 2012;8:649–652. doi: 10.1038/nphys2373. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

