Extra uterine development of preterm kidneys
- PMID: 29500630
- PMCID: PMC5943378
- DOI: 10.1007/s00467-018-3899-1
Extra uterine development of preterm kidneys
Abstract
Objective: We carried out a study to determine the impact of prematurity on renal development. The primary outcomes measured were nephrinuria and albuminuria; renal volume and glomerular filtration rate were the secondary outcomes.
Methods: Preterm neonates born at less than 28 weeks of gestation, with birth weight between 10th and 90th centile (appropriate for gestational age), were recruited and underwent assessments at 28, 32 and 37 weeks postmenstrual age (PMA).
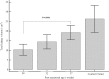
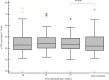
Results: Fifty-three premature neonates and 31 term neonates (control) were recruited. The median gestational age of the premature neonates was 26.4 [24.7-27.4] weeks, with a mean birth weight of 886 (179) g. The mean gestational age of term neonates was 39.1 (1.2) weeks and the mean birth weight was 3406 (406) g. The median age of the term neonates was 6.5 [3.0-12.5] days. The total kidney volume (TKV) almost doubled from 10.3 (2.9) cm3 at 28 weeks PMA to 19.2 (3.7) cm3 at 37 weeks PMA (P = 0.0001). TKV at 37 weeks PMA was significantly smaller compared to term neonates (19.2 (3.7) vs 26.3 (7.0) cm3; P = 0.0001). However, there was no significant difference in estimated glomerular filtration rate (eGFR) between premature neonates (at 37 weeks PMA) and term neonates (control) (43.5 [39.7-48.9] vs. 42.0 [38.2-50.0] mL/min/1.73 m2; P = 0.75). There was a statistically significant decline in nephrin-creatinine ratio and albumin-creatinine ratio from 32 to 37 weeks PMA.
Conclusions: Despite having a smaller renal volume (and fewer nephrons), extremely premature neonates achieve similar eGFRs at corrected term as term-born neonates, likely through single nephron hyperfiltration. Extremely premature neonates also show evidence of glomerular injury.
Keywords: Estimated glomeruli filtration rate; Premature; Preterm; Renal volume.
Conflict of interest statement
Ethics statement
The Townsville Health District Human Research Ethics Committee approved this study, which was conducted following the tenets of the Declaration of Helsinki. Written consent was obtained from parents of all neonates who participated in this study.
Conflict of interests
The authors declare that they have no conflict of interest.
Figures
References
-
- Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, Tong X, Lv H, Ding Y, Liu F, Xu P, Liu W, Cheng H, Chen T, Zeng S, Jia W, Li Z, Qiu H, Wang J, Feng Z. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;16:174. doi: 10.1186/s12887-016-0716-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous