Innate Immune Signaling and Alcohol Use Disorders
- PMID: 29500721
- PMCID: PMC6120815
- DOI: 10.1007/164_2018_92
Innate Immune Signaling and Alcohol Use Disorders
Erratum in
-
Correction to: Innate Immune Signaling and Alcohol Use Disorders.Handb Exp Pharmacol. 2018;248:621. doi: 10.1007/164_2018_193. Handb Exp Pharmacol. 2018. PMID: 30810863
Abstract
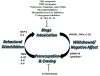
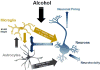
Innate immune signaling is an important feature in the pathology of alcohol use disorders. Alcohol abuse causes persistent innate immune activation in the brain. This is seen in postmortem human alcoholic brain specimens, as well as in primate and rodent models of alcohol consumption. Further, in vitro models of alcohol exposure in neurons and glia also demonstrate innate immune activation. The activation of the innate immune system seems to be important in the development of alcohol use pathology, as anti-immune therapies reduce pathology and ethanol self-administration in rodent models. Further, innate immune activation has been identified in each of the stages of addiction: binge/intoxication, withdrawal/negative affect, and preoccupation/craving. This suggests that innate immune activation may play a role both in the development and maintenance of alcoholic pathology. In this chapter, we discuss the known contributions of innate immune signaling in the pathology of alcohol use disorders, and present potential therapeutic interventions that may be beneficial for alcohol use disorders.
Keywords: Addiction; Alcohol; Neuroimmune; Treatment.
Figures
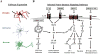


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

