Efficacy of Propranolol, Bisoprolol, and Pyridostigmine for Postural Tachycardia Syndrome: a Randomized Clinical Trial
- PMID: 29500811
- PMCID: PMC6095784
- DOI: 10.1007/s13311-018-0612-9
Efficacy of Propranolol, Bisoprolol, and Pyridostigmine for Postural Tachycardia Syndrome: a Randomized Clinical Trial
Abstract
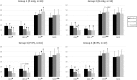
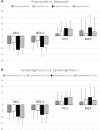
Postural tachycardia syndrome (POTS) is a form of dysautonomia which presents with complex symptoms including orthostatic intolerance. Several medications are prescribed for POTS; however, the efficacy of sustained medical treatment has not been well-investigated. Here, we conducted a 2 × 2 factorial design, randomized, clinical trial of a 3-month medical treatment regimen in POTS patients. Patients were randomly allocated to 4 treatment groups (Group 1: propranolol; Group 2: bisoprolol; Group 3: propranolol + pyridostigmine; Group 4: bisoprolol + pyridostigmine). The orthostatic intolerance questionnaire (OIQ), Beck depression inventory-II (BDI-II), and short-form health survey (SF-36) were conducted at baseline, 1 and 3 months after treatment. Seventy-seven patients who completed the 3-month follow-up were analyzed. In total, every clinical score improved significantly after medical treatment. The OIQ score was significantly lower than that at baseline (18.5 ± 6.7) after 1 month (12.5 ± 4.5, P < 0.01), which decreased further after 3 months (7.8 ± 5.7, P < 0.01). The OIQ score improvements were consistent across every treatment group. In the subgroup analysis of 59 patients who did not receive antidepressants, the BDI-II score significantly decreased after treatment, regardless of the regimen. Physical components of the SF-36 improved after 3 months in every group, while mental components improved only in Group 3. The amount of changes in each score was similar among groups throughout the comparisons. Sustained medical treatment is beneficial to POTS patients, not only for orthostatic intolerance symptoms but also for depression and diminished quality of life, even without prescriptions for antidepressants. The efficacy of each regimen in POTS patients was comparable.
Trial registration: NCT02171988.
Keywords: Bisoprolol; Postural tachycardia syndrome; Propranolol; Pyridostigmine; Randomized trial.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
A prospective, 1-year follow-up study of postural tachycardia syndrome.Mayo Clin Proc. 2012 Aug;87(8):746-52. doi: 10.1016/j.mayocp.2012.02.020. Epub 2012 Jul 15. Mayo Clin Proc. 2012. PMID: 22795533 Free PMC article.
-
Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience.Pacing Clin Electrophysiol. 2011 Jun;34(6):750-5. doi: 10.1111/j.1540-8159.2011.03047.x. Epub 2011 Mar 16. Pacing Clin Electrophysiol. 2011. PMID: 21410722
-
Short-term efficacy of ORS formulation and propranolol regimen in children with POTS.Arch Pediatr. 2020 Aug;27(6):328-332. doi: 10.1016/j.arcped.2020.06.001. Epub 2020 Jul 7. Arch Pediatr. 2020. PMID: 32651146 Clinical Trial.
-
Pyridostigmine in the treatment of orthostatic intolerance.Ann Pharmacother. 2007 Feb;41(2):314-8. doi: 10.1345/aph.1H458. Epub 2007 Feb 6. Ann Pharmacother. 2007. PMID: 17284509 Review.
-
Postural tachycardia syndrome: a heterogeneous and multifactorial disorder.Mayo Clin Proc. 2012 Dec;87(12):1214-25. doi: 10.1016/j.mayocp.2012.08.013. Epub 2012 Nov 1. Mayo Clin Proc. 2012. PMID: 23122672 Free PMC article. Review.
Cited by
-
Benefits and Risks of Medications Used in the Management of Hypotension: A Review.Cureus. 2024 Jan 3;16(1):e51608. doi: 10.7759/cureus.51608. eCollection 2024 Jan. Cureus. 2024. PMID: 38313995 Free PMC article. Review.
-
Long-Term Effects of COVID-19.Mayo Clin Proc. 2022 Mar;97(3):579-599. doi: 10.1016/j.mayocp.2021.12.017. Epub 2022 Jan 12. Mayo Clin Proc. 2022. PMID: 35246288 Free PMC article. Review.
-
Acute Water Ingestion as a Treatment for Postural Orthostatic Tachycardia Syndrome.J Innov Card Rhythm Manag. 2019 Feb 15;10(2):3541-3544. doi: 10.19102/icrm.2019.100206. eCollection 2019 Feb. J Innov Card Rhythm Manag. 2019. PMID: 32477718 Free PMC article.
-
Postural Orthostatic Tachycardia Syndrome (POTS): A critical assessment.Prog Cardiovasc Dis. 2020 May-Jun;63(3):263-270. doi: 10.1016/j.pcad.2020.03.010. Epub 2020 Mar 25. Prog Cardiovasc Dis. 2020. PMID: 32222376 Free PMC article. Review.
-
Efficacy of β-Blockers on Postural Tachycardia Syndrome in Children and Adolescents: A Systematic Review and Meta-Analysis.Front Pediatr. 2019 Nov 7;7:460. doi: 10.3389/fped.2019.00460. eCollection 2019. Front Pediatr. 2019. PMID: 31788462 Free PMC article.
References
-
- Sheldon RS, Grubb B, 2nd, Olshansky B, Shen W-K, Calkins H, Brignole M, et al. 2015 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Treatment of Postural Tachycardia Syndrome, Inappropriate Sinus Tachycardia, and Vasovagal Syncope. Heart rhythm: the official journal of the Heart Rhythm Society. 2015;12(6):e41–63. doi: 10.1016/j.hrthm.2015.03.029. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

