Peptide identifications and false discovery rates using different mass spectrometry platforms
- PMID: 29501178
- PMCID: PMC5839655
- DOI: 10.1016/j.talanta.2018.01.062
Peptide identifications and false discovery rates using different mass spectrometry platforms
Abstract
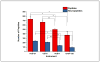
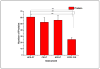
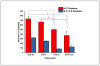
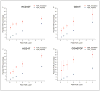
Characterization of endogenous neuropeptides produced from post-translational proteolytic processing of precursor proteins is a demanding task. A variety of complex prohormone processing steps generate molecular diversity from neuropeptide prohormones, making in silico neuropeptide discovery difficult. In addition, the wide range of endogenous peptide concentrations as well as significant peptide complexity further challenge the structural characterization of neuropeptides. Liquid chromatography-mass spectrometry (MS), performed in conjunction with bioinformatics, allows for high-throughput characterization of peptides. Mass analyzers and molecular dissociation techniques render specific characteristics to the acquired data and thus, influence the analysis of the MS data using bioinformatic algorithms for follow-up peptide identification. Here we evaluated the efficacy of several distinct peptidomic workflows using two mass spectrometers, the Thermo Orbitrap Fusion Tribrid and Bruker Impact HD UHR-QqTOF, for confident peptide discovery and characterization. We compared the results in several categories, including the numbers of identified peptides, full-length mature neuropeptides among all identifications, and precursor proteins mapped by the identified peptides. We also characterized the peptide false discovery rate (FDR) based on the occurrence of amidation, a known post-translational modification (PTM) that has been shown to require the presence of a C-terminal glycine. Thus, amidation events without a preceding glycine were considered false-positive amidation assignments. We compared the FDR calculated by the search engine used here to the minimum FDR estimated via false amidation assignments. The search engine severely underestimated the rate of false PTM assignments among the identified peptides, regardless of the specific MS platform used.
Keywords: Amidation; De novo sequencing; Neuropeptides; Peptidomics; Proteomics.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

References
-
- Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity–A review. Neuropeptides. 2006;40:375–401. - PubMed
-
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. - PubMed
-
- De Souza EB. Corticotropin-releasing factor receptors: Physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

