Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum
- PMID: 29501384
- PMCID: PMC6626543
- DOI: 10.1016/j.jid.2018.02.023
Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum
Abstract
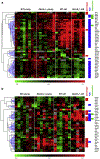
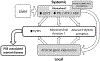
Pseudoxanthoma elasticum (PXE) is a rare genetic condition primarily caused by hepatic ABCC6 transporter dysfunction. Most clinical manifestations of PXE are due to premature calcification of elastic fibers. However, the vascular impact of PXE is pleiotropic and remains ill defined. ABCC6 expression has recently been associated with cellular nucleotide export. We studied the impact of ABCC6 deficiency on blood levels of adenosine triphosphate and related metabolites and on soluble nucleotidase activities in PXE patients and Abcc6-/- mice. In addition, we investigated the expression of genes encoding ectocellular purinergic signaling proteins in mouse liver and aorta. Plasma adenosine triphosphate and pyrophosphate levels were significantly reduced in PXE patients and in Abcc6-/- mice, whereas adenosine concentration was not modified. Moreover, 5'-nucleotidase/CD73 activity was increased in the serum of PXE patients and Abcc6-/- mice. Consistent with alterations of purinergic signaling, the expression of genes involved in purine and phosphate transport/metabolism was dramatically modified in Abcc6-/- mouse aorta, with much less impact on the liver. ABCC6 deficiency causes impaired vascular homeostasis and tissue perfusion. Our findings suggest that these alterations are linked to changes in extracellular nucleotide metabolism that are remote from the liver. This opens new perspectives for the understanding of PXE pathophysiology.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures

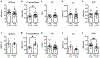

References
-
- Aessopos A, Savvides P, Stamatelos G, Rombos I, Tassiopoulos T, Karagiorga M, et al. Pseudoxanthoma elasticum-like skin lesions and angioid streaks in beta-thalassemia. Am J Hematol 1992;41:159e64. - PubMed
-
- Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem 2003;51:887e902. - PubMed
-
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause Pseudoxanthoma elasticum. Nat Genet 2000;25:228e31. - PubMed
-
- Boraldi F, Annovi G, Bartolomeo A, Quaglino D. Fibroblasts from patients affected by Pseudoxanthoma elasticum exhibit an altered PPi metabolism and are more responsive to pro-calcifying stimuli. J Dermatol Sci 2014a;74: 72e80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

