C-type natriuretic peptide functions as an innate neuroprotectant in neonatal hypoxic-ischemic brain injury in mouse via natriuretic peptide receptor 2
- PMID: 29501420
- PMCID: PMC6087427
- DOI: 10.1016/j.expneurol.2018.02.016
C-type natriuretic peptide functions as an innate neuroprotectant in neonatal hypoxic-ischemic brain injury in mouse via natriuretic peptide receptor 2
Abstract
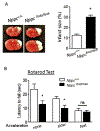
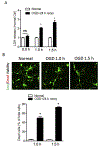
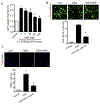
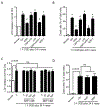
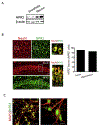
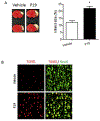
Neonatal hypoxia-ischemia (HI) is the most common cause of brain injury in neonates, which leads to high neonatal mortality and severe neurological morbidity in later life (Vannucci, 2000; Volpe, 2001). Yet the molecular mechanisms of neuronal death and brain damage induced by neonatal HI remain largely elusive. Herein, using both in vivo and in vitro models, we determine an endogenous neuroprotectant role of c-type natriuretic peptide (CNP) in preserving neuronal survival after HI brain injury in mouse pups. Postnatal day 7 (P7) mouse pups with CNP deficiency (Nppclbab/lbab) exhibit increased brain infarct size and worsened long-term locomotor function after neonatal HI compared with wildtype control (Nppc+/+). In isolated primary cortical neurons, recombinant CNP dose-dependently protects primary neurons from oxygen-glucose deprivation (OGD) insult. This neuroprotective effect appears to be mediated through its cognate natriuretic peptide receptor 2 (NPR2), in that antagonization of NPR2, but not NPR3, exacerbates neuronal death and counteracts the protective effect of CNP on primary neurons exposed to OGD insult. Immunoblot and confocal microscopy demonstrate the abundant expression of NPR2 in neurons of the neonatal brain and in isolated primary cortical neurons as well. Moreover, similar to CNP deficiency, administration of NPR2 antagonist P19 via intracerebroventricular injection prior to HI results in exacerbated neuronal death and brain injury after HI. Altogether, the present study indicates that CNP and its cognate receptor NPR2 mainly expressed in neurons represent an innate neuroprotective mechanism in neonatal HI brain injury.
Keywords: C-type natriuretic peptide; Natriuretic peptide receptor 2; Neonatal hypoxic-ischemic brain injury; Neuronal death; Oxygen-glucose deprivation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
C-Type Natriuretic Peptide Ameliorates Vascular Injury and Improves Neurological Outcomes in Neonatal Hypoxic-Ischemic Brain Injury in Mice.Int J Mol Sci. 2021 Aug 20;22(16):8966. doi: 10.3390/ijms22168966. Int J Mol Sci. 2021. PMID: 34445671 Free PMC article.
-
Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats.Neurobiol Dis. 2016 May;89:202-12. doi: 10.1016/j.nbd.2016.02.011. Epub 2016 Feb 11. Neurobiol Dis. 2016. PMID: 26875527 Free PMC article.
-
Natriuretic peptide receptors regulate cytoprotective effects in a human ex vivo 3D/bioreactor model.Arthritis Res Ther. 2013 Jul 24;15(4):R76. doi: 10.1186/ar4253. Arthritis Res Ther. 2013. PMID: 23883591 Free PMC article.
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
-
Hypoxic-ischemic injury in neonatal brain: involvement of a novel neuronal molecule in neuronal cell death and potential target for neuroprotection.Int J Dev Neurosci. 2008 Feb;26(1):93-101. doi: 10.1016/j.ijdevneu.2007.08.013. Epub 2007 Sep 7. Int J Dev Neurosci. 2008. PMID: 17936538 Free PMC article. Review.
Cited by
-
C-type natriuretic peptide preserves central neurological function by maintaining blood-brain barrier integrity.Front Mol Neurosci. 2022 Oct 4;15:991112. doi: 10.3389/fnmol.2022.991112. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36267701 Free PMC article.
-
MicroRNA210 Suppresses Mitochondrial Metabolism and Promotes Microglial Activation in Neonatal Hypoxic-Ischemic Brain Injury.Cells. 2025 Aug 5;14(15):1202. doi: 10.3390/cells14151202. Cells. 2025. PMID: 40801634 Free PMC article.
-
MicroRNA-210 Downregulates TET2 (Ten-Eleven Translocation Methylcytosine Dioxygenase 2) and Contributes to Neuroinflammation in Ischemic Stroke of Adult Mice.Stroke. 2023 Mar;54(3):857-867. doi: 10.1161/STROKEAHA.122.041651. Epub 2023 Feb 3. Stroke. 2023. PMID: 36734233 Free PMC article.
-
Atrial Natriuretic Peptide Promotes Neurite Outgrowth and Survival of Cochlear Spiral Ganglion Neurons in vitro Through NPR-A/cGMP/PKG Signaling.Front Cell Dev Biol. 2021 Jun 23;9:681421. doi: 10.3389/fcell.2021.681421. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34268307 Free PMC article.
-
3D doppler ultrasound imaging of cerebral blood flow for assessment of neonatal hypoxic-ischemic brain injury in mice.PLoS One. 2023 May 9;18(5):e0285434. doi: 10.1371/journal.pone.0285434. eCollection 2023. PLoS One. 2023. PMID: 37159455 Free PMC article.
References
-
- Andoh T, Chock PB, Chiueh CC, 2002. Preconditioning-mediated neuroprotection: role of nitric oxide, cGMP, and new protein expression. Ann. N. Y. Acad. Sci 962, 1–7. - PubMed
-
- Beaudoin GM 3rd, et al., 2012. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc 7, 1741–1754. - PubMed
-
- Bennett BD, et al., 1991. Extracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antisera. J. Biol. Chem 266, 23060–23067. - PubMed
-
- Bolouri H, et al., 2014. Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann. Neurol 75, 395–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

