Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats
- PMID: 29501528
- PMCID: PMC6119551
- DOI: 10.1016/j.neuropharm.2018.02.033
Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats
Abstract
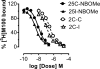
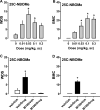
2,5-Dimethoxyphenethylamines (2C compounds) are 5-HT2A/2C receptor agonists that induce hallucinogenic effects. N-methoxybenzylation of 2C compounds markedly increases their affinity for 5-HT2A receptors, and two such analogs, 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe), have emerged in recreational drug markets. Here, we investigated the neuropharmacology of 25C-NBOMe and 25I-NBOMe in rats, as compared to their 2C analogs and the prototypical 5-HT2A/2C agonist 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine (DOI). Compounds were tested in vitro using 5-HT2A receptor binding and calcium mobilization assays. For in vivo experiments, 25C-NBOMe (0.01-0.3 mg/kg), 25I-NBOMe (0.01-0.3 mg/kg), 2-(4-chloro-2,5-dimethoxyphenyl)ethanamine (2C-C) (0.1-3.0 mg/kg), 2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (2C-I) (0.1-3.0 mg/kg) and DOI (0.03-1.0 mg/kg) were administered subcutaneously (sc) to male rats, and 5-HT2A-mediated behaviors were assessed. NBOMes displayed higher affinity for 5-HT2A receptors than their 2C counterparts but were substantially weaker in functional assays. 25C-NBOMe and 25I-NBOMe were much more potent at inducing wet dog shakes (WDS) and back muscle contractions (BMC) when compared to 2C-C and 2C-I. Pretreatment with the selective 5-HT2A antagonist (R)-(2,3-dimethoxyphenyl){1-[2-(4-fluorophenyl)ethyl]-4-piperidinyl}methanol (M100907) reversed behaviors produced by all agonists. Interestingly, binding affinities at the 5-HT2A receptor were significantly correlated with potencies to induce BMC but not WDS. Our findings show that NBOMes are highly potent 5-HT2A agonists in rats, similar to effects in mice, and consistent with the reported hallucinogenic effects in human users. This article is part of the Special Issue entitled 'Psychedelics: New Doors, Altered Perceptions'.
Keywords: 5-HT(2A) receptor; Back muscle contractions; NBOMe; Wet dog shakes.
Published by Elsevier Ltd.
Figures




References
-
- Baumann MH, Rothman RB. Chronic cocaine exposure potentiates prolactin and head shake responses to 5-HT2 receptor stimulation in rats. Neuropharmacology. 1996;35:295–301. - PubMed
-
- Bedard P, Pycock CJ. "Wet-dog" shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. - PubMed
-
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

