Supramolecular self assembly of nanodrill-like structures for intracellular delivery
- PMID: 29501722
- PMCID: PMC6008205
- DOI: 10.1016/j.jconrel.2018.02.041
Supramolecular self assembly of nanodrill-like structures for intracellular delivery
Abstract
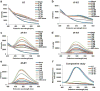
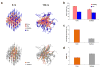
Despite recent advances in the supramolecular assembly of cell-penetrating peptide (CPP) nanostructures, the tuning of size, shape, morphology and packaging of drugs in these materials still remain unexplored. Herein, through sequential ligation of peptide building blocks, we create cell-penetrating self-assembling peptide nanomaterials (CSPNs) with the capability to translocate inside cells. We devised a triblock array of Tat48-59 [HIV-1 derived transactivator of transcription48-59] based CPPs, conjugated to up to four Phenylalanine (Phe) residues through an amphiphilic linker, (RADA)2. We observed that the sequential addition of Phe leads to the transition of CSPN secondary structures from a random coil, to a distorted α-helix, a β-sheet, or a pure α-helix. This transition occurs due to formation of a heptad by virtue of even number of Phe. Atomic force microscopy revealed that CSPNs form distinct shapes reminiscent of a "drill-bit". CSPNs containing two, three or four Phe, self-assemble into "nanodrill-like structures" with a coarse-twisted, non-twisted or fine-twisted morphology, respectively. These nanodrills had a high capacity to encapsulate hydrophobic guest molecules. In particular, the coarse-twisted nanodrills demonstrate higher internalization and are able to deliver rapamycin, a hydrophobic small molecule that induced autophagy and are capable of in vivo delivery. Molecular dynamics studies provide microscopic insights into the structure of the nanodrills that can contribute to its morphology and ability to interact with cellular membrane. CSPNs represent a new modular drug delivery platform that can be programmed into exquisite structures through sequence-specific fine tuning of amino acids.
Keywords: Cell penetrating peptides; Intracellular delivery; Nanodrills; Supramolecular assembly.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

