Design, synthesis, and biological evaluation of stable β6.3-Helices: Discovery of non-hemolytic antibacterial peptides
- PMID: 29501941
- PMCID: PMC8366898
- DOI: 10.1016/j.ejmech.2018.02.057
Design, synthesis, and biological evaluation of stable β6.3-Helices: Discovery of non-hemolytic antibacterial peptides
Abstract
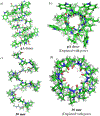
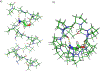
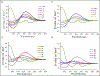
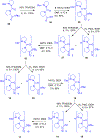
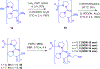
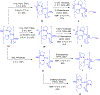
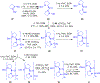
Gramicidin A, a topical antibiotic made from alternating L and D amino acids, is characterized by its wide central pore; upon insertion into membranes, it forms channels that disrupts ion gradients. We present helical peptidomimetics with this characteristic wide central pore that have been designed to mimic gramicidin A channels. Mimetics were designed using molecular modeling focused on oligomers of heterochiral dipeptides of proline analogs, in particular azaproline (AzPro). Molecular Dynamics simulations in water confirmed the stability of the designed helices. A sixteen-residue Formyl-(AzPro-Pro)8-NHCH2CH2OH helix was synthesized as well as a full thirty-two residue Cbz-(AzPro-Pro)16-OtBu channels. No liposomal lysis activity was observed suggesting lack of channel formation, possibly due to inappropriate hydrogen-bonding interactions in the membrane. These peptidomimetics also did not hemolyze red blood cells, unlike gramicidin A.
Keywords: Antibiotic; Azaproline foldamers; Gramicidin; Liposomes and beta-helices; RBC hemolysis.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Figures






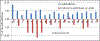

References
-
- Cabezas E, Satterthwait AC, The hydrogen bond mimic approach: solid-phase synthesis of a peptide stabilized as an α-helix with a hydrazone link, J. Am. Chem. Soc, 121 (1999) 3862–3875.
-
- Chapman RN, Dimartino G, Arora PS, A highly stable short α-helix constrained by a main-chain hydrogen-bond surrogate, J. Am. Chem. Soc, 126 (2004) 12252–12253. - PubMed
-
- Stanger HE, Gellman SH, Rules for antiparallel β-sheet design: D-Pro-Gly is superior to L-Asn-Gly for β-hairpin Nucleation, J. Am. Chem. Soc, 120 (1998) 4236–4237.
-
- Schneider JP, Kelly JW, Templates that induce α-helical, β-sheet, and loop conformations, Chem. Rev, 95 (1995) 2169–2187.
-
- Kemp DS, Bowen BR, Muendel CC, Synthesis and conformational analysis of epindolidione-derived peptide models for β-sheet formation, J. Org. Chem, 55 (1990) 4650–4657.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

